Abstract
Background. Diabetic nephropathy (DN) is a severe long-term complication of diabetes characterized by continuous albuminuria, a relentless decline in renal function, and an increased arterial blood pressure.
Aims. Our aim was to find out if single nucleotide polymorphisms (SNPs) within the SLC22A1, SLC22A2, and SLC22A3 genes encoding organic cation transporters (OCTs) associate with DN or hypertension.
Subjects and methods. We selected 90 SNPs (≈1 SNP/4 kb) in and surrounding SLC22A1, SLC22A2, and SLC22A3 using the HapMap data. The SNPs were tested for association with DN and hypertension in 1,086 unrelated Finnish patients with type 1 diabetes mellitus (T1DM). Eight of the SNPs were genotyped in 1,252 additional Finnish patients to verify the findings.
Results. We detected nominal evidence of association (P < 0.05) between the SLC22A2 (SNPs rs653753, rs596881, and rs316019) and SLC22A3 (SNPs rs376563, rs2048327, rs2457576, and rs1567438) genes and DN and hypertension in Finnish men with T1DM. We were not, however, able to replicate the associations, and none of them reached the significance limit adjusted for multiple testing (P < 0.00009).
Conclusions. There was no clear association between the SLC22A1, SLC22A2, and SLC22A3 genes and DN or hypertension. Although several SLC22A2 and SLC22A3 SNPs indicated association, lack of association was evident after the replication study.
| Abbreviations | ||
| AER | = | albumin excretion rate |
| BMI | = | body mass index |
| DBP | = | diastolic blood pressure |
| DN | = | diabetic nephropathy |
| eGFR | = | estimated glomerular filtration rate |
| HWE | = | Hardy-Weinberg equilibrium |
| LD | = | linkage disequilibrium |
| MAF | = | minor allele frequency |
| OCT | = | organic cation transporter |
| OMIM | = | Online Mendelian Inheritance in Man |
| SBP | = | systolic blood pressure |
| SLC22A1 | = | gene encoding solute carrier family 22 (organic cation transporter), member 1 |
| SLC22A2 | = | gene encoding solute carrier family 22 (organic cation transporter), member 2 |
| SLC22A3 | = | gene encoding solute carrier family 22 (organic cation transporter), member 3 |
| SNP | = | single nucleotide polymorphism |
| T1DM | = | type 1 diabetes mellitus |
Key messages
Elevated blood pressure is a major risk factor for the development and progression of diabetic nephropathy (DN). Based on the available evidence, the SLC22A1, SLC22A2, and SLC22A3 genes encoding organic cation transporters are relevant candidate genes for both hypertension and DN.
Despite nominal evidence of association between the SLC22A2 and SLC22A3 single nucleotide polymorphisms (SNPs) and DN and hypertension in Finnish men with type 1 diabetes mellitus in the original sample set, the findings could not be confirmed in the replication study.
Introduction
Diabetic nephropathy (DN) is a severe long-term complication of diabetes characterized by continuous albuminuria, a relentless decline in renal function, and an increased arterial blood pressure. It occurs as a result of a complex interplay between still largely unknown genetic as well as environmental factors (Citation1,Citation2).
Organic cation transporters OCT1 (OMIM #602607), OCT2 (OMIM #602608), and OCT3 (OMIM #604842), also known as solute carrier family 22 members 1-3 (SLC22A1-3), are encoded by the SLC22A1, SLC22A2, and SLC22A3 genes located as a cluster on chromosome 6q26-q27. In human, SLC22A1 is expressed mainly in the liver, whereas SLC22A2 is expressed most strongly in the kidneys but also in the brain. SLC22A3 has a much more wide-spread expression pattern including the small intestine, liver, kidneys, placenta, skeletal muscle, heart, and brain. OCTs are essential for the homeostasis of a number of physiologically important endogenous cations including catecholamines, prostaglandins, and agmatine. They are also necessary for the renal clearance of a broad range of substrates including toxins, xenobiotics, and common pharmaceuticals (e.g. metformin and β-blockers) (Citation3).
The renal OCT expression and organic cation transport are significantly reduced in experimental diabetes (Citation4,Citation5) and in humans with diabetes (Citation6). This may be physiologically important since 15%–20% of the total clearance of monoamine neurotransmitters (including noradrenaline, adrenaline, and dopamine) occurs via OCT-dependent pathways (Citation7), potentially contributing to increased levels of circulating catecholamines (Citation8), hypertension, and proteinuria in diabetes (Citation9). Furthermore, increased levels of prostacyclin are associated with hyperfiltration and renal injury in the early stages of DN (Citation10,Citation11), and impaired tubular uptake of the antiproliferative cation agmatine may contribute to fibrosis and advanced glycation end-product (AGE) accumulation in the diabetic kidney (Citation12). Consistent with these hypotheses, a microsatellite marker on chromosome 6q27, in relative proximity to the SLC22A1-3 genes, has been associated with DN in type 1 diabetes mellitus (T1DM) (Citation13) and linked to essential hypertension (Citation14). There is also evidence that a single nucleotide polymorphism (SNP) within SLC22A2 is associated with essential hypertension (Citation15). Moreover, Slc22a3-deficient mice show an increased preference for hypertonic saline under thirst and salt appetite conditions (Citation16), indicating a role for OCT3 in salt-intake regulation.
Based on the available evidence, the genes encoding OCTs are considered relevant candidate genes for both DN and hypertension. To find out whether SNPs within the SLC22A1, SLC22A2, and SLC22A3 genes associate with DN or hypertension, we performed a comprehensive case-control association study in Finnish patients with T1DM.
Subjects and methods
Study subjects
We performed a candidate gene association study in unrelated Finnish patients with T1DM (). Sample set 1 (n = 1,086) was applied for primary association analyses and set 2 (n = 1,252) for replication. The patients were recruited from all over Finland as part of the Finnish Diabetic Nephropathy (FinnDiane) Study. We also genotyped 165 non-diabetic Finns recruited nationwide. The blood samples of these population controls as well as information on age, sex, and place of blood donation were provided by the Finnish Red Cross Blood Service. The study was approved by the ethics committees of each participating center. All patients gave a written informed consent to participate.
Table I. Clinical characteristics of the patients with type 1 diabetes mellitus stratified by normo-, micro-, and macroalbuminuria.
The diagnosis of T1DM was based on age at onset <35 years, permanent insulin treatment initiated within 1 year of the diagnosis, and a fasting C-peptide level <0.3 nmol/L. The renal status was based on urinary albumin excretion rate (AER), and patients were stratified into those with normal AER (<20 μg/min or <30 mg/24 h in at least two out of three consecutive overnight or 24-h urine collections), microalbuminuria (AER ≥20 and <200μg/min or ≥30 and <300 mg/24 h), or macroalbuminuria (i.e. nephropathy; AER ≥200 μg/min or ≥300 mg/24 h) (). Patients with normal AER were required to have diabetes duration of at least 15 years to ensure the renal status. Patients on dialysis or those who had received a kidney transplant, i.e. end-stage renal disease (ESRD) patients, were not included in this study.
Comprehensive data on each patient's medical history and life-style were collected, and a clinical examination was performed. Blood and urine samples were taken for measurement of C-peptide, HbA1c, AER, and serum creatinine. Estimated glomerular filtration rate (eGFR) was calculated using the Cockcroft-Gault formula (Citation17) adjusted for body surface area. Blood pressure was measured as previously described in detail (Citation18), and hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or use of antihypertensive medication. Hypertension was used as a dichotomous variable in the genetic association analyses since many of the patients were taking antihypertensive medication () and blood pressure measurement data per se would not have represented the untreated naive blood pressure status.
Power calculations
We calculated the power of this study to detect a genetic association with DN using the Genetic Power Calculator available at http://pngu.mgh.harvard.edu/∼purcell/gpc/ (Citation19). We set nephropathy prevalence to 0.3 (among patients with T1DM); equal high-risk and marker allele frequencies; complete linkage disequilibrium (LD) between high-risk and marker alleles (D’ = 1); and relative risk of 1.6 for homozygous and 1.3 for heterozygous high-risk allele carriers (additive model). For set 1 (363 patients with macroalbuminuria, i.e. cases, and 453 patients with normal AER, i.e. controls), 80% power was reached with a marker allele frequency of 0.16 (P = 0.05). For set 2 (154 cases and 963 controls), 79% power was achieved with a marker allele frequency of 0.4. A marker allele frequency of 0.07 was sufficient to give >80% power for set 1 and set 2 together (517 cases and 1,416 controls).
SNP selection
Initially we selected 36 SNPs from the SLC22A1, SLC22A2, and SLC22A3 genes using the HapMap data (http://www.hapmap.org/) (Citation20) and the Haploview program v.3.11 (http://www.broad.mit.edu/mpg/haploview/about.php) (Citation21). Subsequently, we performed a more comprehensive screen and used the program Tagger (http://www.broad.mit.edu/mpg/haploview/about.php) (Citation22) to select tag SNPs. We took the sequence ranging from 160,498,000 to 160,862,000 bp (364 kb) from the HapMap database (HapMap Data Rel#20/phaseII Jan06, on NCBI 35 assembly, dbSNP b125) and downloaded the SNPs genotyped in the HapMap CEU sample (U.S. residents of northern and western European ancestry) (Citation20). By taking into account the previously genotyped 36 SNPs, 63 tag SNPs were selected using the pair-wise tagging option of Tagger. Of a total of 99 SNPs, 6 SNPs (rs568567, rs3119312, rs533452, rs628031, rs598135, and rs624249) were excluded due to poor genotyping performance, and 93 SNPs were genotyped in set 1 (Supplementary Table I). Eight of these SNPs were selected for a replication study based on preliminary association findings, LD, minor allele frequency (MAF), and Sequenom multiplexing capacity.
Genotyping
Genomic DNA was extracted from peripheral blood using the standard phenol-chloroform method (Citation23) or PUREGENE( DNA Purification Kit (Gentra Systems, Inc., Minneapolis, MN, USA). The original 36 SNPs were genotyped using the TaqMan® chemistry (Citation24) implemented on the ABI Prism® 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) in a single SNP format. The remaining 57 SNPs and the 8 SNPs replicated in set 2 were genotyped using the Homogeneous MassExtend MassARRAY system (Sequenom, San Diego, CA, USA) (Citation25) in a multiplex format. Both methods have excellent success (>95%) and accuracy (100%) rates in our hands (Citation26).
Statistical analyses
We tested the SNPs for deviation from Hardy-Weinberg equilibrium (HWE) in the non-diabetic subjects as well as in the patients with T1DM using the chi-square test. Single SNP associations with DN and hypertension were tested using the chi-square test. SPSS v.15.0 for Windows (SPSS, Inc., Chicago, IL, USA) and the Haploview program v.4.0 were used for these analyses. Pair-wise LD measures (D’ and r2) were calculated and LD patterns examined using the confidence interval algorithm (Citation27) integrated in Haploview. Haplotype association analyses were also performed using the Haploview program.
Significance limits
To account for all the SNPs and phenotypes tested, we applied a robust correction for multiple testing. We calculated the number of independent SNPs using the equation proposed by Li and Ji (Citation28) and the Single Nucleotide Polymorphism Spectral Decomposition (SNPSpD) program available at http://gump.qimr.edu.au/general/daleN/SNPSpD/ (Citation29). The 90 SNPs tested for association corresponded to 46.4 independent SNPs. When this is multiplied by 12 for the number of phenotype variables (DN and hypertension) and subgroups analyzed, the number of tests sums to 557. The adjusted significance limit for this study is thus P < 0.00009 (0.05/557).
Results
SNP characteristics
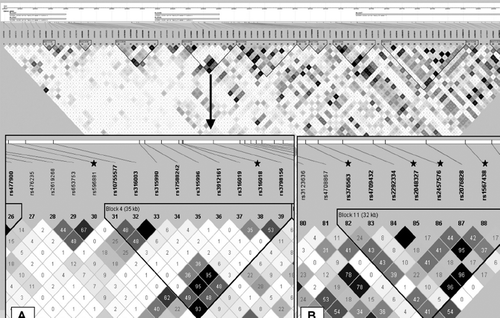
We tested the SLC22A1, SLC22A2, and SLC22A3 SNPs for association with DN and hypertension in Finnish patients with T1DM. Clinical characteristics of the patients are summarized in . We genotyped 93 SNPs within the genes, intergenic regions, and ≈15 kb up- and down stream of the genes. Two of the SNPs (rs9457843 within SLC22A1 and rs578560 between SLC22A2 and SLC22A3) deviated from HWE (P < 0.001) in the non-diabetic Finnish subjects and were excluded from the association analyses. The remaining SNPs were in HWE both in the non-diabetic subjects as well as in the patients with T1DM (data not shown). MAFs of the SNPs ranged from 0.05 to 0.5 in the non-diabetic individuals, and these were similar to those of the HapMap CEU sample (Supplementary Table I). The only exception was the SNP rs6937722 within SLC22A1 (MAF 0.02 in the Finns) that was excluded. Consequently, 90 SNPs (≈1 SNP/4 kb) were tested for association with DN and hypertension. Out of these SNPs, 82 were HapMap tag SNPs capturing 96% of the common SNPs (MAF ≥0.05) in the studied region at r2 ≥ 0.8 in the HapMap population with European ancestry. The LD pattern of the region is shown in .
Figure 1. Pair-wise linkage disequilibrium (LD) and haplotype block structure for the 90 SLC22A1, SLC22A2, and SLC22A3 SNPs covering 364 kb in 165 non-diabetic Finnish subjects. The absolute position of each SNP is shown below the genes in the upper panel of the figure. Pair-wise r2 values are represented within the boxes (when no number is given r2=1). The white-to-black color scheme represents increasing strength of LD. The zoomed-in figures show the SLC22A2 (A) and SLC22A3 (B) gene regions, with six out of the eight SNPs replicated in set 2 being marked with stars. Haplotype block structure was determined using the confidence interval algorithm integrated in the Haploview program.
Genetic association with DN
We compared allele frequencies and genotype distributions for the SLC22A1, SLC22A2, and SLC22A3 SNPs between the patients with normal AER (n = 453) and with macroalbuminuria (n = 363) (set 1). Patients with microalbuminuria were excluded because approximately one-third of them will regress to normoalbuminuria and one-third will progress to macroalbuminuria (Citation30). No statistically significant differences (P < 0.05) were observed between the groups, and this was also the case when the association was tested in men and women separately (data not shown).
We subsequently divided the patients into those with HbA1c < 8.4% (median for the normo- and macroalbuminuric patients in set 1; 8.3% in set 2) and those with HbA1c ≥ 8.4% and tested the allele and genotype association with DN in these subgroups and also in men and women separately. The only evidence of association was observed in men with HbA1c ≥ 8.4% (132 cases and 74 controls). The SNPs from rs376563 to rs2063346, covering a 32.5-kb region at the 3’ end of SLC22A3 (), showed evidence of allele and genotype association (P-values ranging from 0.004 to 0.04) with DN (Supplementary Table II). There was also evidence of haplotype association (P = 0.04; data not shown). Since several neighboring SNPs showed evidence of association (although none of the associations reached the significance limit adjusted for multiple testing, P < 0.00009), we tried to confirm the findings in order not to lose any real association due to too strict a significance limit. We genotyped six SLC22A3 SNPs (rs377551, rs316244, rs376563, rs2048327, rs2457576, and rs1567438) in set 2 (including 52 macroalbuminuric and 217 normoalbuminuric men with HbA1c ≥ 8.4%), but no statistically significant differences were observed. Detailed data for the SNPs rs376563, rs2048327, rs2457576, and rs1567438 showing the strongest associations are shown in . Although more significant associations were observed when the two sample sets were analyzed jointly (184 patients with macroalbuminuria and 291 patients with normal AER), none of the associations reached the adjusted significance limit (P < 0.00009).
Table II. Detailed association data for the selected SLC22A3 and SLC22A2 SNPs.
There was also some evidence of association between the SLC22A1 SNPs and DN and hypertension (Supplementary Table II), but none of the associations reached the adjusted significance limit (P < 0.00009). Based on the association data for SLC22A2 and SLC22A3 with several SNPs indicating association, the gene expression patterns (see Introduction), and a previously reported association between the SLC22A2 SNP rs316019 and essential hypertension (Citation15), we decided to focus on the associations with the SLC22A2 and SLC22A3 SNPs.
Genetic association with hypertension
We compared allele frequencies and genotype distributions between the patients with (n = 751) and without (n = 330) hypertension (set 1) and performed the analyses also in men (423 cases and 134 controls) and women (328 cases and 196 controls) separately. No statistically significant associations were observed in the whole sample set or in women (data not shown), but seven SLC22A3 SNPs showed evidence of association (P-values ranging from 0.01 to 0.04) with hypertension in men (Supplementary Table II). Particularly, association was observed at three consecutive SNPs (rs2457576, rs2076828, and rs1567438) covering 14 kb at the 3’ end of SLC22A3 (Supplementary Table II, ). To verify these findings the SNPs rs2457576 (D’ = 1 with rs2076928) and rs1567438 were genotyped in set 2 including 332 hypertensive and 292 normotensive men, but no statistically significant differences were observed (). More significant associations were observed when the two sample sets were combined (755 cases and 426 controls), but none of the associations reached the adjusted significance limit (P < 0.00009).
In addition to the SLC22A3 SNPs, we found three SNPs (rs653753, rs596881, and rs316019) covering 43.4 kb at the 3’ end of SLC22A2 () showing allele, genotype (P = 0.03; Supplementary Table II), and haplotype association (P-values ranging from 0.03 to 0.04; data not shown) with hypertension in men. The SNP rs596881 (D’ = 0.91 with rs653753) was further genotyped in set 2 in addition to rs316018 that is in complete LD (D’ = 1) with rs316019. The SNP rs316019 was not genotyped in set 2 due to its lower MAF. None of the associations in set 2 or in the combined sample set reached the adjusted significance limit (P < 0.00009).
Discussion
We did not observe clear association between the SLC22A1, SLC22A2, and SLC22A3 genes and DN or hypertension. Although several SLC22A2 and SLC22A3 SNPs indicated association in Finnish men with T1DM, lack of association was evident after replication in another sample set, and the significance limit adjusted for multiple testing was not reached. It is thus possible that the positive findings are spurious and due to the small sample size of the subgroups in which the associations were observed. On the other hand, the adjusted significance limit may be too stringent since it is based on the assumption of independency of the tests although there is a strong correlation, e.g. between DN and hypertension.
Moreover, the data by Lazar et al. speaks for the association showing that the SLC22A2 SNP rs316019 associates with essential hypertension in Caucasian patients with cardiovascular disease (Citation15). The association observed by Lazar et al. was even stronger in patients with diabetes and in men (Citation15). In our study, a few SLC22A2 SNPs, including rs316019, indicated association with hypertension in men although the significance limit adjusted for multiple testing was not reached. An explanation for the associations being observed only in men or being stronger in men could be that the genetic variants have different effects on the phenotype in males and females. This may in turn be due to some intrinsic or even environmental factors. For example, OCT2 expression has been shown to be higher in male than in female rats, mice, and dogs due to testosterone (Citation3). It could thus be hypothesized that higher testosterone levels in men might potentiate the detrimental effects of at least the SLC22A2 variants. However, one should be cautious about extrapolating transport-related sex differences between species.
In this study several SLC22A3 SNPs indicated evidence of association with DN in the subgroup of men with poor glycemic control, although the associations did not survive multiple testing, whereas Lazar et al. showed stronger association between SLC22A2 and essential hypertension in patients with diabetes (Citation15). Previous studies have also shown evidence of association with DN subsequent to patients being stratified by HbA1c (Citation31,Citation32). These subgroup analyses are clinically justified since poor glycemic control is a major risk factor for DN (Citation33). Such grouping of patients may increase the difference in high-risk allele frequencies between cases and controls and thus improve the power for a given sample size. Moreover, the blood glucose concentration and OCT expression and function may be associated since it has been suggested that AGEs and non-enzymatic glycation of OCTs have an effect on tubular transport of organic cations (Citation4,Citation5).
The SLC22A1, SLC22A2, and SLC22A3 genes are relevant candidate genes for hypertension and nephropathy in diabetes since elevated blood pressure is a major risk factor for the development and progression of DN (Citation34). Moreover, it has been suggested that the nephropathy risk in T1DM is associated with a genetic predisposition to hypertension, particularly in patients with poor glycemic control (Citation35). In the kidneys, impaired expression or function of OCTs might interfere with the dopamine homeostasis and modulate sodium excretion and blood pressure regulation thereby predisposing to hypertension and nephropathy. Intriguingly, it has been shown that a deficiency in the renal dopamine production or dopamine receptor dysfunction can contribute to the development of various forms of hypertension (Citation36,Citation37). Furthermore, SLC22A2 and SLC22A3 are both expressed in the brain, and OCT2 and OCT3 participate in the regulation of interstitial and intracellular concentrations of dopamine and other monoamine neurotransmitters (Citation3,Citation38). OCT3 has also been suggested to be essential for correct behavioral responses to environmentally induced variations in osmolarity (Citation16). Impaired expression or function of OCTs might thus interfere with the aminergic neurotransmission in the brain and regulation of salt and water intake, which could in turn affect blood pressure.
All the patients and non-diabetic subjects in this study were of Finnish descent. The SNPs were selected using the data for the HapMap CEU sample and are likely to capture most of the common variation within the studied region since the HapMap tag SNPs have been shown to transfer well across the Caucasian populations, including the Finns (Citation39). It should be noted, however, that we may have failed to identify rare susceptibility variants and those of small effect sizes. To verify the possible association between the SLC22A2 and SLC22A3 genes and DN and hypertension, replication in a larger sample set, resequencing of the region, and functional studies are required.
Supplementary Material
Download PDF (171.9 KB)Acknowledgements
We thank warmly all the patients participating in the FinnDiane Study. We acknowledge the assistance of the whole FinnDiane Study Group (Citation40) and, especially, the skilled technical assistance of Maija Parkkonen, Sinikka Lindh, and Anna Sandelin. SNP genotyping based on the Sequenom technology was performed by the Finnish Genome Center.
Declaration of interest: This study was funded by the Academy of Finland (214335, 124280 to MW), the Sigrid Juselius Foundation, the Diabetes Research Foundation (to MW), the Liv och Hälsa Foundation, the Folkhälsan Research Foundation, the Research Funds of the Helsinki University Central Hospital, the Wilhelm and Else Stockmann Foundation, and the European Commission (contract no. QLG2-CT-2001-01669).
References
- Harjutsalo V, Katoh S, Sarti C, Tajima N, Tuomilehto J. Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes. 2004;53: 2449–54.
- Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am. 2004;88:1001–36.
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24: 1227–51.
- Thomas MC, Tikellis C, Kantharidis P, Burns WC, Cooper ME, Forbes JM. The role of advanced glycation in reduced organic cation transport associated with experimental diabetes. J Pharmacol Exp Ther. 2004;311:456–66.
- Grover B, Buckley D, Buckley AR, Cacini W. Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes. J Pharmacol Exp Ther. 2004;308: 949–56.
- Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, Piechota HJ, . Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–84.
- Martel F, Ribeiro L, Calhau C, Azevedo I. Comparison between uptake2 and rOCT1: effects of catecholamines, metanephrines and corticosterone. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:303–9.
- De Mattia G, Ferri C, Laurenti O, Cassone-Faldetta M, Piccoli A, Santucci A. Circulating catecholamines and metabolic effects of captopril in NIDDM patients. Diabetes Care. 1996;19:226–30.
- Hoogenberg K, Sluiter WJ, Navis G, Van Haeften TW, Smit AJ, Reitsma WD, . Exogenous norepinephrine induces an enhanced microproteinuric response in microalbuminuric insulin-dependent diabetes mellitus. J Am Soc Nephrol. 1998;9:643–54.
- DeRubertis FR, Craven PA. Eicosanoids in the pathogenesis of the functional and structural alterations of the kidney in diabetes. Am J Kidney Dis. 1993;22:727–35.
- Makino H, Tanaka I, Mukoyama M, Sugawara A, Mori K, Muro S, . Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–65.
- Marx M, Trittenwein G, Aufricht C, Hoeger H, Lubec B. Agmatine and spermidine reduce collagen accumulation in kidneys of diabetic db/db mice. Nephron. 1995;69: 155–8.
- McKnight AJ, Maxwell AP, Sawcer S, Compston A, Setakis E, Patterson CC, . A genome-wide DNA microsatellite association screen to identify chromosomal regions harboring candidate genes in diabetic nephropathy. J Am Soc Nephrol. 2006;17:831–6.
- Caulfield M, Munroe P, Pembroke J, Samani N, Dominiczak A, Brown M, . Genome-wide mapping of human loci for essential hypertension. Lancet. 2003;361:2118–23.
- Lazar A, Zimmermann T, Koch W, Grundemann D, Schomig A, Kastrati A, . Lower prevalence of the OCT2 Ser270 allele in patients with essential hypertension. Clin Exp Hypertens. 2006;28:645–53.
- Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci. 2004;24:2846–51.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Ronnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH, . Altered age-related blood pressure pattern in type 1 diabetes. Circulation. 2004;110:1076–82.
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–50.
- International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–320.
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
- de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23.
- Vandenplas S, Wiid I, Grobler-Rabie A, Brebner K, Ricketts M, Wallis G, . Blot hybridisation analysis of genomic DNA. J Med Genet. 1984;21:164–72.
- Livak KJ, Marmaro J, Todd JA. Towards fully automated genome-wide polymorphism screening. Nat Genet. 1995;9: 341–2.
- Jurinke C, van den Boom D, Cantor CR, Koster H. Automated genotyping using the DNA MassArray technology. Methods Mol Biol. 2002;187:179–92.
- Lahermo P, Liljedahl U, Alnaes G, Axelsson T, Brookes AJ, Ellonen P, . A quality assessment survey of SNP genotyping laboratories. Hum Mutat. 2006;27:711–4.
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, . The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9.
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005; 95:221–7.
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–9.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–93.
- Pettersson-Fernholm K, Forsblom C, Hudson BI, Perola M, Grant PJ, Groop PH, . The functional -374 T/A RAGE gene polymorphism is associated with proteinuria and cardiovascular disease in type 1 diabetic patients. Diabetes. 2003;52:891–4.
- Lindholm E, Bakhtadze E, Sjogren M, Cilio CM, Agardh E, Groop L, . The -374 T/A polymorphism in the gene encoding RAGE is associated with diabetic nephropathy and retinopathy in type 1 diabetic patients. Diabetologia. 2006;49: 2745–55.
- Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–5.
- Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, . Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105.
- Krolewski AS, Canessa M, Warram JH, Laffel LM, Christlieb AR, Knowler WC, . Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:140–5.
- Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–47.
- Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood). 2003;228:134–42.
- Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96.
- Lundmark PE, Liljedahl U, Boomsma DI, Mannila H, Martin NG, Palotie A, . Evaluation of HapMap data in six populations of European descent. Eur J Hum Genet. 2008;16:1142–50.
- Saraheimo M, Forsblom C, Thorn L, Waden J, Rosengard-Barlund M, Heikkila O, . Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care. 2008;31:1165–9.