Abstract
Background. Nebivolol is a highly selective β1-adrenoreceptor antagonist with vasodilating properties. This study investigated its effect on quality of life (QoL) and blood pressure (BP) in real life conditions. In total, 1468 patients were enrolled, 12% diabetics. Nebivolol was prescribed as monotherapy, add-on or switch medication. Methods. In this open-label, prospective study, the JNC-VII BP target values were used: <140/90 and <130/80 mmHg for diabetics. The responder rate and the QoL was determined at baseline and after 4 and 8 weeks. Results. After 4 weeks, 27% of subjects reached target BP, 45% after 8 weeks. The responder rates were 92, 90 and 83% for the monotherapy, add-on and switch groups. Compared with baseline, all showed statistical significance at 8 weeks. Similarly to results for the QoL after 8 weeks, the mean improvement in QoL for all three groups was 9–10 points (total range: 0–88). Conclusions. The study demonstrates that nebivolol in mild to moderate hypertension is associated with overall improvements in QoL, with a marked BP-lowering effect, in mono-therapy, add-on or switch, irrespective of the glucose tolerance status. It may be hypothesized that its dual mode of action explains its BP-lowering effect as well as the tolerability
Introduction
All therapeutically applied β-adrenoreceptor blocking agents are known to block β1-adrenoreceptors, by which they slow the heart rate and therefore reduce blood pressure (BP). Various beta-blockers, however, also substantially block β2-adrenoreceptors, leading to adverse effects, such as broncho- and/or vasoconstriction. The ESH/ESC 2007 Guidelines, as well as the Fourth Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice, no longer recommend beta-blockers as first choice BP-lowering drugs because of their diabetogenic and dysmetabolic effects in subjects with the metabolic syndrome phenotype [Citation1,Citation2]. As stated in these updated guidelines, these effects seem, however, less pronounced or absent in beta-blockers with intrinsic vasodilating properties such as carvedilol and nebivolol [Citation1,Citation2].
Endothelial-derived factors modulate the contractile activity of smooth muscle cells in the vascular wall, and nitric oxide (NO) [Citation3,Citation4], produced and released from the endothelial cells, induces vessel wall relaxation and reduces vascular resistance [Citation5,Citation6]. Nebivolol is a highly selective b -blocking agent that combines beta-blocking and vasodilating properties [Citation4,Citation7]. The haemodynamic profile of nebivolol allows effective control of BP at a low degree of beta-blockade, while maintaining left ventricular function [Citation8].
Subjects with mild to moderate hypertension are in general without complaints [Citation9]. Since antihypertensive drugs have to be taken for many years, it is extremely important that BP-lowering therapies minimally interfere with subject's quality of life (QoL) so that drug compliance in the long run remains high [Citation10]. Among beta-blockers, nebivolol's efficacy, safety and tolerability were ranked high in several studies [Citation11–15], whose size and design, however, were not specifically aimed at assessing the drug's effects on a subject's QoL. The goal of this study was therefore to assess, in a population-based cohort of subjects in real life conditions, with mild to moderate hypertension, the QoL and efficacy of nebivolol at different intervention levels, according to the Seventh report of the Joint National committee (JNC-VII) on Prevention, Detection, Evolution and Treatment of High Blood Pressure [Citation16].
Subjects and methods
This was an open-label, prospective, multicentre study designed to test the hypothesis that preservation of QoL can be maintained at a significantly higher level by the use of nebivolol, alone or in combination with other treatment(s), compared with other therapeutic options, with similar anti-hypertensive effect. One hundred and thirty-three GPs in Belgium enrolled 1468 patients (>18 years old) with essential hypertension and unsatisfactory control of BP at inclusion [seated diastolic BP (DBP) ≥95 and <115 mmHg] or complaining of intolerable adverse events during prior treatment.
Exclusion criteria included secondary hypertension, severe hypertension [systolic BP (SBP) >200 and/or DBP ≥115 mmHg], prior nebivolol therapy, hypersensitivity or contra-indication to beta-blockers, and major concomitant illnesses. The study was conducted in compliance with the Helsinki Declaration of 1975 (as revised in 1983) and approved by the Ethics Committee of the Clinique St-Etienne, Brussels. All patients provided a written informed consent prior to enrolment.
The study protocol included three visits. At the screening visit (V1), anthropometric data, BP, including hypertension duration and complications (stroke, transient ischaemic attack, angina, myocar-dial infarction, cardiac insufficiency, left ventricular hypertrophy) and heart rate were collected. Also, creatininaemia, associated conventional cardiovascular risk factors, dietary habits, and the practitioner's decision regarding high BP therapy (addition of, or switch to nebivolol (or other drug) for drug-naïve subjects or for subjects already receiving hypotensive drug(s)). Visits 2 and 3 (V2 and V3) were scheduled 4 and 8 weeks after V1. At these follow-up visits, the following data were collected: BP, heart rate, type and dose of hypotensive drug(s). Office systolic and diastolic BPs were measured after 5 min in sitting position, always on the same arm.
The study aim was to improve BP levels using a pharmacological intervention in order to reach JNC-VII recommended values for BP control (<140/90 mmHg for non-diabetic subjects, and <130/80 mmHg for diabetic subjects). The physician could freely decide between several alternatives: (i) to administer nebivolol to patients who were not taking any BP medication at the time (monotherapy group), (ii) to add nebivolol to existing BP-lowering agent(s) (add-on group), or (iii) to replace one of the anti-hypertensive drug(s) with nebivolol (switch group). Only one anti-hypertensive medication could be changed at V1, thereafter, the chosen treatment remained throughout the study period. The medication was prescribed by the physician at visit 1 and purchased by the subjects. The BP-lowering efficacy of nebivolol was ascertained from the proportion of controlled subjects, i.e. reaching JNC-VII recommended values for BP in non-diabetic (140–90 mmHg) and diabetic (130–80 mmHg) subjects prior to and following nebivolol treatment, as well as the proportion of responders. Responders were defined as subjects in whom systolic BP values decreased by ≥10 mmHg and diastolic BP decreased by ≥5 mmHg while on nebivolol, irrespective of JNC-VII target thresholds attainment, and/or those who considered as BP controlled at V2 and V3.
All participants filled out the QoLaN questionnaire at each visit (). This questionnaire was designed for self-completion and consisted of 22 items, each with five possible answers pertaining to the previous month's subjective assessment of wellness as well as of items related to high BP symptoms and/or of frequently reported side-effects of commonly used BP-lowering drugs [Citation17,Citation18].
Table I. Questionnaire.
Statistical analysis
Both quantitative and qualitative aspects of the observed changes in the QoL questionnaire were analysed in order to assess the significance of changes. The differences between means (V3 and V1) for each of the 22 QoL items were first analysed using paired Student's t-test within each group. One-way analysis of variance was used to compare V3 and V1 means, as well as differences between the three groups. Statistical significance was also inferred from longitudinal changes in the absolute and relative distribution of answers to the QoLaN questionnaire, using symmetry distribution analysis.
For comparisons between V3 and V1, only patients for whom both data points were available were included. Results were considered significant at the 5% critical level. Calculations were carried out using the SAS (version 9.1 for Windows) statistical package.
Results
The total number of subjects included in the study was 1468, with 587 subjects in the monotherapy group (nebivolol as sole BP-lowering agent), 460 subjects in the add-on group (nebivolol added to existing BP-lowering agent(s)) and 421 subjects in the switch group (a previous BP-lowering agent switched to nebivolol) (). In all three groups, a high proportion of subjects was overweight and had truncal fat distribution as reflected by enlarged waist circumference. At baseline, mean systolic BP values were well above the JNC-VII recommended cut-off values for BP control.
Table II. Baseline characteristics.
The group of BP-lowering drug-naïve subjects given nebivolol as monotherapy were more recently diagnosed with high BP than those in the add-on (n=460) or switch (n=421) groups (1 vs. 7 years). The sex ratio was well balanced in each group. Systolic and diastolic BP values were similar in the monotherapy and the add-on groups, and lower (by a mean 10 and 7 mmHg) in the switch group. In the latter group, 15% of subjects were within normal JNC-VII target at inclusion, vs. 1% in the drug-naïve and add-on groups. Mean arterial pressure was also lower (±10 mmHg) in the switch group. Known diabetes prevalence was 5% (monotherapy), 20% (add-on) and 12% (switch).
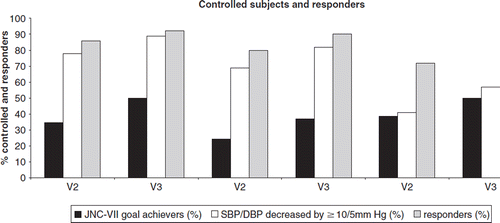
The commonest reason for switching or introducing nebivolol was inadequate BP control; this was mentioned for 73, 64 and 26% of cases in the monotherapy, add-on and switch groups, respectively. In the monotherapy and add-on groups, side-effects, tiredness and headache were mentioned for 9–5% of cases. For the switch group, side-effects came close to unsatisfactory BP control (24 vs. 26%), followed by tiredness, cold-extremities and headache (12, 11 and 5%, respectively). The responder and JNC-VII goal achievement rates are described in . At V3, an overall 45% of subjects under nebivolol therapy were adequately controlled (vs 5% at inclusion). This incremental 40% was achieved through a 27% increase at V2 followed by a further 13% increase of controlled subjects at V3. In each group, similar trends were observed between V2 and V3 with respect to the proportions of subjects deemed adequately controlled.
Figure 1. Proportion (%) of controlled subjects and responders under nebivolol in each treatment category (monotherapy, add-on, switch) at visits 2 and 3: proportion of JNC-VII goal achievers (dark column), proportion of subjects with SBP/DBP decreased by more than 10/5 mmHg (white column), and proportion of responders defined by SBP/DBP decreased by more than 10/5 mmHg irrespective of JNC-VII target thresholds attainment and/or those who were considered as BP controlled (grey column)
The results of the QoLaN questionnaire were analysed both quantitatively and qualitatively ( and ). The analyses were performed for total scoring and also for subscores representing series of items that were grouped according to: (i) the physical aspects (tiredness; dizziness; facial redness; cold hands and/or feet; coughing; tinnitus; breathing difficulty; palpitations; headache; and swollen legs and/or feet), (ii) the subjective feelings (anxiety; satisfaction with one's personal life; depression; satisfaction with one's family life; satisfaction in performing one's daily activities; satisfaction with one's professional life) and (iii) the symptomatic status (climbing a flight of stairs; walking around the block several times; giving up one's usual leisure time activities; having to take leave from work for medical reasons over the previous month; being satisfied with one's sex life and satisfaction with one's current hyper-tension treatment), as assessed by the questionnaire. For the qualitative component of the analysis, the outcome between V3 and V1 was categorized as status quo, worsening or improvement. Another way to qualitatively assess the changes was to compute, for each subject, the total number of items improved, which ranges between 0 (no item improved) and 22 (all items improved), and to compare these numbers between the three treatment groups.
Table III. Quantitative changes in Quality of life items.
Table IV. Qualitative changes in Quality of life (QoL) items.
At baseline, the mean (±SD) total score of the 22 questionnaire items (range 0–88) was 24.1±14.4 (monotherapy), 25.5±13.5 (add-on), and 25.4±13.4 (switch). The mean total difference in (at the bottom of the right column) was calculated by attributing a negative value to all improved values; in doing so, the differences between V3 and V1 for items 12, 14, 15, 16, 21 and 22, for which a positive value means improvement (because of the manner in which the original questions were formulated), were converted to a negative value. This results in mean improvements of –9.67, –9.15 and –9.52 in the three groups. The scores dropped markedly and significantly in each group (p<0.0001), with no differences between groups (). The number of items improved per patient was not statistically different between the three groups (7.2, 7.3 and 7.0; p=0.74) ().
Scoring at inclusion significantly differed between groups with respect to the following QoL items: cold hands and/or feet; palpitations; headache; swollen legs and/or feet; climbing a flight of stairs; walking around the block several times; and satisfaction with one's sex life. These differences were in line with the expected rationale(s) for introducing (or switching to) nebivolol, and/or with drug-related side-effects in the already treated group. For the following items, scoring at final visit (V3; after 2 months on nebivolol) significantly differed between groups: climbing a flight of stairs; walking around the block several times; giving up one's usual leisure time activities; and being satisfied with one's sex life. With respect to the magnitude of changes in QoL scoring between inclusion and termination, the following items significantly differed between groups: cold hands and/or feet; palpitations; headache; and anxiety. The latter effects were in keeping with nebivolol's dual pharmacological properties (). The three specific subscores (physical aspects, subjective feelings, and symptomatic status) also dropped significantly within each group (p<0.0001; not shown).
The qualitative changes between V3 and V1 within each nebivolol group (monotherapy, add-on and switch) are detailed in . The table lists the proportions of subjects reporting improvement, worsening or lack of change for each QoLaN questionnaire component between V3 and V1. These items relate either to QoL or clinically relevant items, as potential rationale(s) for introducing/ switching BP-lowering drug(s) and/or as side-effects associated with common classes of BP-lowering drugs. All groups showed harmonious drifts toward improvement, except for items 4 (cold hands and/or feet), 9 (headache), 11 (anxiety) and 15 (satisfaction in performing daily activities), for which the changes were different between groups. At V3, all items showed ample shifts in the sliding scale distribution toward improvement of QoL, lesser side-effects or both. These shifts were obvious in the different groups (monotherapy, add-on and switch), with the most marked differences observed for tiredness, palpitations, headache, and cold extremities.
The questionnaire's items that shifted most were those with the largest absolute difference in improvement vs. worsening (such as tiredness, headache, palpitations, satisfaction with current therapy), i.e. items for which the proportion of subjects reporting “no change” between V3 and V1 was the lowest. While absenteeism only modestly shifted toward improvement in the overall cohort and in the monotherapy and switch groups, there was a slight shift toward increased absenteeism in the add-on group. As a whole, the scale shifts in the overall cohort were paralleled in the groups, both in magnitude and directionality. Regarding cold extremities, however, the improvement-to-worsening ratio was more pronounced in the switch group ().
Discussion
The QoLaN study assessed, in a population-based cohort of subjects with mild to moderate hypertension, the QoL and efficacy of nebivolol at different intervention levels of the JNC-VII hypertension treatment algorithm. With respect to JNC-VII BP thresholds for diabetic and non-diabetic subjects, nebivolol use was highly effective as monotherapy, as add-on or as switch agent, as indicated by the percentages of goal-achieving subjects during and at the end of the study. In order to enumerate the responders, we applied strict criteria, namely a combined systolic/diastolic decrement of ≥10/5 mmHg. Not withstanding the level of intervention (monotherapy, add-on, switch), nebivolol use entailed that the majority of subjects reached the criteria to be categorized as belonging to the responder category at study completion, irrespective of glucose tolerance status. Furthermore, the results of this study are in line with the concept evoked in the ESH/ESC 2007 Guidelines regarding specificities within the beta-blockers class [Citation1,Citation2].
The design of the QoLaN questionnaire aimed at documenting wellness while being specifically adapted to capture the magnitude of both common hypertension symptoms and/or commonly reported side-effects of the usual BP-lowering drugs in a population-based study. As reported by Van Bortel et al. [Citation14], nebivolol use is particularly neutral with respect to overall tolerance to various levels of exercise, and this may account for part of the benefits reported. Thus, the improvements in questionnaire's items were related not only to the named side-effects or to conditions commonly observed following the use of first-generation beta-blockers, but also to more holistic matters pertaining to personal, family of professional life, as well as satisfaction with current therapy.
The QoLaN population included a high proportion of subjects with a known diagnosis of diabetes mellitus, in whom strict BP control is essential to prevent micro- and macrovascular complications. The finding of high diabetes prevalence was not unexpected, since insulin resistance and the metabolic syndrome phenotype are common underlying conditions and co-morbidities in subjects with essential hypertension and/or type 2 diabetes, the common form of diabetes mellitus. The ample BP-lowering efficacy of nebivolol reported in this study was observed both in non-diabetic and diabetic subjects, despite the latter having to reach more ambitious BP goals to qualify as responders. The uniform results observed in non-diabetic and diabetic subjects suggest that nebivolol may be a well-tolerated means of decreasing BP through beta-blockade regardless of glucose homeostasis status. This may be of particular interest in those diabetic subjects requiring beta-blockade while suffering of lower limbs arterial macroangiopathy.
This study has obvious limitations, including its open design as an open phase IV study (where any change in treatment may result in subjective improvement) with substantial and unsurprising dropout rate. However, the baseline characteristics of non-completers were not different from completers (not shown). Another potential limitation is the use of a specifically designed, though unvalidated, questionnaire to assess the effects of nebivolol. On the other hand, the chosen questions were designed to capture any relevant changes in physical aspects, subjective feelings and symptomatic status, which altogether may significantly impact on first-line management of high BP in daily life. A potential confounder of the QoLaN questionnaire resides in its lack of allowance for current employment (vs. retirement) status. Considering the average age of the study population (61 years), one may reasonably assume that a large proportion of subjects who reported no change were actually retired. The open study design also has inherent limitations. On the other hand, the real-life setting and the magnitude of the cohort size contribute to relevance of the observed changes. Furthermore, no previous large-scale study addressed the QoL issue with respect to using a dual-effect beta-blocker such as nebivolol in primary care as BP-lowering agent.
As a class, beta-blockers are highly effective BP-lowering drugs, albeit with a higher degree of class-related side-effects compared with newer renin– angiotensin blocking agents. Yet, in a double-blind, randomized, parallel group study evaluating nebivolol and angiotensin receptor blocker losartan on QoL and BP-lowering, Van Bortel et al. [Citation19] found similar decrease in SBP, a larger decrease in DBP with nebivolol and no difference in overall QoL assessing physical well-being. The QoLaN study questionnaire addressed not only aspects of physical well-being, but also subjective feelings as well as the impact of current health status on daily activities.
In conclusion, the QoLaN study found in a population-based cohort with mild to moderate hypertension that nebivolol markedly improves both BP and QoL at different intervention levels of the JNC-VII hypertension treatment algorithm. These improvements were of similar magnitude whenever nebivolol was introduced as a monotherapy, add-on or switch treatment modality in a mixed non-diabetic and diabetic population in a primary care setting.
Acknowledgement
This study was funded by a grant of Menarini Benelux SA/NV.
Disclosure: Prof. Dr. M. Hermans has received speaker's fees, advisory honoraria and/or scientific or travel grants from: AstraZeneca, Aventis, Bayer, Covance, Danone, E. Lilly, Solvay Pharma, GSK, LifeScan, Lipha, Medtronic-Minimed, Menarini Benelux, Menarini Diagnostics, Menarini International, MSD, MSH, NovoNordisk, Nycomed, Pfizer, Roche, Sanofi-Aventis, Servier Benelux, Solvay Pharma. Prof. Dr. A. Albert and L. Seidel carried out the statistical analysis and their department was paid by Menarini Benelux for the work done. Prof. Dr. P. van de Borne: no conflicts of interest to report. Dr. O. De Coster has received speaker's fees, advisory honorary and travel grants from: Boston Scientific, Biotronik, Medtronic, Solvay Pharma, Novartis, AstraZeneca, Menarini Benelux and Servier Benelux.
References
- The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 Guidelines for the Management of Arterial Hypertension. J Hypertens. 2007; 25:1105–1187.
- Fourth Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice. Cardiovascular Disease Prevention in Clinical Practice (European Guidelines on). Eur J Cardiovasc Prev Rehab. 2007; 14Suppl. 2:S1–S113.
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri E. Endothelium derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987; 84:9265–9269.
- Ignarro LJ, Byrns RE, Trinh K, Sisodia M, Buga GM. Nebivolol: a selective beta(1)-adrenergic receptor antag onist that relaxes vascular smooth muscle by nitric oxide- and cyclic GMP-dependent mechanisms. Nitric Oxide. 2002; 7:75–82.
- Cockcroft JR, Chowienczyk PJ, Brett SE, Chen CP, Dupont AG, Van Nueten L, Wooding SJ, Ritter JM. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995; 274:1067–1071.
- Van Bortel LM, Van Merode T, Smeets FA. Nebivolol improves the vessel wall properties of the common carotid artery. Drug Invest. 1991; 3:61–63.
- Cockcroft JR. Exploring vascular benefits of endothelium-derived nitric oxide. Am J Hypertens. 2005; 1812 Pt 2:177S–183S.
- Agabiti Rosei E, Rizzoni D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs. 2007; 67:1097–1107.
- Wojciechowski D, Papademetriou V. Beta-blockers in the management of hypertension: focus on nebivolol. Expert Rev Cardiovasc Ther. 2008; 6:471–479.
- Williams GH. Quality of Life and its impact on hypertensive patients. Am J Med. 1987; 82:98–105.
- Ambrosioni E, Borghi C. Tolerability of nebivolol in head-to-head clinical trials versus other cardioselective b-blockers in the treatment of hypertension. High Blood Press Cardiovasc Prev. 2005; 12:27–35.
- Czuriga I, Riecansky I, Bodnar J, Fulop T, Kruzsicz V, Kristof E, Edes I. for The NEBIS Investigators; NEBIS Investigators Group. Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the nebivolol, bisoprolol multicenter study (NEBIS). Cardiovasc Drugs Ther. 2003; 17:257–263.
- Uhliř O, Fejfuš M, Havránek K, Lefflerová K, Vojáček J, Widimskyˇ J, Winterová J, Zeman K. Nebivolol versus metoprolol in the treatment of hypertension. Drug Invest 3. 1991; S1:107–110.
- Van Bortel LM, van Baak MA. Exercise tolerance with nebivolol and atenolol. Cardiovasc Drugs Ther. 1992; 6:239–247.
- Celik T, Yuksel UC, Iyisoy A, Kursaklioglu H, Ozcan O, Kilic S, Ozmen N, Isik E. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J Hypertension. 2006; 24:591–596.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, . National Heart, Lung, and Blood Institute. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003; 289:2560–2572.
- Breed JGS, Ciampricotti R, Tromp UP, Valster PA, Lageweg B, Van Bortel LMAB. Quality of life perception during antihypertensive treatment. A comparative study of bisoprolol and enalapril. J Cardiovasc Pharmacol. 1992; 20:750–755.
- Bulpitt CI, Fletcher AL. The measurement of quality of life in hypertensive patients: a practical approach. Br J Clin Pharmacol. 1990; 30:353–364.
- Van Bortel LM, Bulpitt CJ, Rici F. Quality of life and antihypertensive effect with nebivolol and losartan. Am J Hypertens. 2005; 18:1060–1066.