Abstract
Background. Elevated blood glucose (BG) induced by antihypertensive agents increases the risk of cardiovascular events. This study was designed to investigate whether fosinopril+indapamide combination therapy has any effect on glucose tolerance (GT), and if it did, whether conversion to fosinopril alone could reverse the impaired GT. Methods. Included in the present study were 124 hypertensive patients, of whom 62 patients were treated with fosinopril plus indapamide (F/I group) and the remaining 62 patients were treated with fosinopril alone (F group). Of them, 89 patients completed a mean of 14-month follow-up. In the F/I group, 29 patients were converted to the use of fosinopril for 4–12 months after they completed the follow-up. Results. In the F group, fasting BG decreased significantly from 5.1±0.5 to 4.8±0.7 mmol/l (p<0.01), and 2-h postprandial BG decreased significantly from 7.2±1.6 to 6.4±1.4 mmol/l (p<0.01), while in the F/I group, fasting BG increased significantly from 5.1 ±0.6 to 5.3±0.9 mmol/l (p<0.05), and 2-h postprandial BG increased significantly from 7.2±1.7 to 7.7±1.8 mmol/l (p<0.05). In 29 patients of the F/I group who completed the follow-up and were converted to fosinopril, fasting BG decreased significantly from 5.5±1.0 to 5.3±1.0 mmol/l (p<0.05), and 2-h postprandial BG decreased significantly from 7.5±2.0 to 7.0±2.7 mmol/l (p<0.05). Conclusion. Fosinopril+indapamide combination therapy impaired GT in Chinese hypertensive patients, and fosinopril alone was able to reverse fosinopril+indapamide-induced GT impairment in part of these patients.
Introduction
It is generally accepted that simultaneous administration of two or more antihypertensive medications can control blood pressure (BP) more effectively and tolerably, thus further lowering hypertension-related cardiovascular events (Citation1,Citation2). For this reason, some guidelines for hypertension prevention and treatment advocate combination therapy of antihypertensive agents for the purpose of achieving goal BP (Citation3,Citation4). Newly diagnosed diabetes mellitus (DM) and elevated blood glucose (BG) induced by antihypertensive agents increase the risk of cardiovascular events in hypertensive patients (Citation5,Citation6), and it is therefore of clinical significance to understand the overall effect of antihypertensive agents used for combination therapy on glucose metabolism in hypertensive patients.
Combination use of angiotensin-converting enzyme inhibitors (ACEI) and diuretics is one of the protocols recommended in the guidelines for management of hypertension (Citation3,Citation4), and has been used extensively in hypertensive patients in China. ACEI can reduce the development of new DM onset in hypertensive patients, increase serum potassium and lower serum uric acid (Citation7–9), while diuretics have adverse effects of impairing glucose metabolism, reducing serum potassium and increasing serum uric acid (Citation10–12). It is therefore believed that simultaneous administration of ACEI and diuretics can prevent diuretic-induced adverse effects.
There are controversial reports about indap-amide, a long-acting hypotensor agent with both diuretic and vasodilative actions. Some studies (Citation13,Citation14) reported that indapamide (1.5˜2.5 mg/day) alone in the treatment of hypertension for 2–24 months did not produce significant influence on BG, while other studies (Citation15) reported that indapamide (2.5 mg/day) alone in the treatment of hypertension for 24 weeks significantly raised BG. Whether ACEI+indapamide combination therapy could prevent indapamide-induced glucose tolerance (GT) impairment has been a clinical concern. Compared with thiazide diuretics, there are few reports about indapamide-induced GT impairment. Clinicians in China generally believe that the ACEI+indapamide protocol is the treatment of choice in ACEI+diuretics combination therapy, supposing that this protocol may not cause significant GT impairment in hypertensive patients or even have a favorable effect. However, there is a lack of studies to support this in Chinese hypertensive patients.
It is known that some antihypertensive agents can cause GT impairment (Citation10,Citation16). Nevertheless, there is no prospective study to confirm whether discontinuation of a GT-impairing antihypertensive protocol and initiation of another antihypertensive protocol could reverse impaired GT.
The present study observed the effects of fosinopril+indapamide combination therapy or fosinopril alone on GT in Chinese hypertensive patients. If fosinopril+indapamide combination therapy did impair GT, the patients were converted to fosinopril alone to see whether it could reverse GT impairment induced by fosinopril+indapamide combination therapy.
Methods
Study population
Eligible subjects included those: (i) who lived near Changhai Hospital for the convenience of follow-up; (ii) who had a systolic BP of 140–179 mmHg and/or a diastolic BP of 90–109 mmHg by three consecutive measurements; and (iii) whose body weight (BW) was relatively stable over several months before enrollment. Exclusion criteria included those who had received any treatment for weight reduction, lipid lowering and BG control for a year before enrollment, who had a history of treatment with ACEI and diuretics, and who had the following existing or preexisting diseases: secondary hypertension, DM, gout, myocardial diseases, cardiac valve diseases, a history of cerebral hemorrhage or cerebral infarction, significant liver or kidney dysfunction, or a history of persistent chronic hepatitis, pregnancy, lactation, prolonged administration of contraceptives.
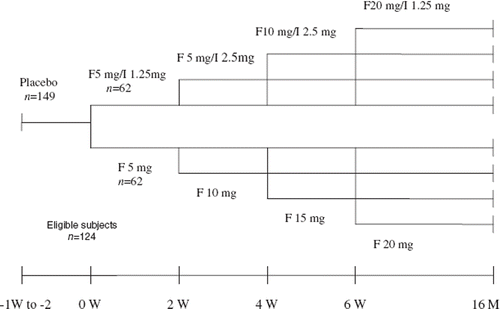
There were 149 subjects who met the preliminary inclusion and exclusion criteria. Of them, 15 patients had a history of intermittent use of antihypertensive medications before enrollment, and after discontinuation of the antihypertensive medications, they were put on placebo treatment for 2 weeks. The remaining 134 patients had no history of using any antihypertensive medication, and they were put on placebo treatment for a week. After physical examination, laboratory evaluation and BP measurement by the end of the placebo treatment, 25 subjects were excluded from the study, eight because of diagnosis of DM and significant liver or kidney dysfunction, and 17 because of failure to meet the original BP inclusion criteria. Of the remaining 124 randomized subjects, 12 had a history of intermittent use of anti-hypertensive medications before enrollment (), and the remaining 112 subjects had no history of using any antihypertensive medication.
Table I. Baseline characteristics of patients enrolled.
Study design
This study was a prospective, randomized, parallel controlled clinical trial. After baseline measurements were completed, 124 eligible subjects were completely randomized to two groups, where 62 patients were treated with fosinopril+indapamide (F/I group) and 62 patients were treated with fosinopril (F group). Patients in the F/I group were started on fosinopril 5 mg four times a day, and indapamide 1.25 mg daily. They were followed up at 2-week intervals to see whether it was necessary to regulate the dosage. If BP failed to descend below 140/90 mmHg, the dosage was increased to the maximum of 20 mg for fosinopril and 2.5 mg for indapamide. Patients in the F group were started on fosinopril 5 mg daily. They were followed up at 2-week intervals to see whether it was necessary to regulate the dosage. If BP failed to descend below 140/90 mmHg, the dosage of fosinopril was increased to the maximum of 20 mg In the first 3 months, the patients were followed up biweekly, and then monthly (). All patients were followed up for 12–16 months with a mean of 14 months. The laboratory data by the end of follow-up showed that fosinopril+indapamide combination therapy impaired GT. Therefore, 29 patients in the F/I group consented to discontinue the use of indapamide and continue with the treatment with fosinopril alone for another 4–12 months (mean 6 months). During the follow-up period, the patients were asked not to change the dietary habits and physical activities, and not to receive any weight, glucose, lipid and uric acid reduction therapies or other antihypertensive treatments.
The study protocol was approved by the ethics committees of all participating institutions and written informed consent was obtained from each patient.
BW, body mass index (BMI), waist circumference (WC) and waist-to-hip ratio (WHR) measurement
The patients were measured for body height and BW without clothes, shoes and caps on before eating breakfast before treatment, at 6 months and by the end of treatment. BMI was calculated. WC and hip circumferences were measured at the middle level between the lower costal margin and the anterior superior iliac spine and at the level of femoral tuber-osity respectively. WHR was calculated.
Measurement of BP and HR
After the patients had been sitting at rest for 10 minin the outpatient clinic, BP in sitting position was measured with a standard mercury-column sphyg-momanometer. The Korotkoff first sound was taken as systolic BP (SBP) and the fifth sound as diastolic BP (DBP). The measurement was repeated three times with 5-min intervals and the average value was used. HR was measured by cardiac auscultation for 60 s immediately after BP measurements.
Metabolic parameters
Following items were measured for all patients: (i) a 75-g oral GT test (OGTT) was performed at 07:00 h for glucose and insulin, and the area under the curve of glucose (AUCG) was calculated; and (ii) at the same time serum triglyceride, high-density lipo-protein cholesterol (HDL-C), serum potassium (K), serum uric acid (UA) were measured. By the end of follow-up, OGTT, K and UA were re-examined.
Insulin sensitivity was determined by OGTT based on the formula described by Matsuda & DeFronzo and named as insulin sensitivity index composite [ISI (composite)], which has been shown to correlate well with insulin sensitivity evaluated using clamp technique (Citation17).
According to the WHO standards, normal GT (NGT): fasting BG<7 mmol/l and OGTT 2-h BG<7.8 mmol/l, impaired GT (IGT): fasting BG<7 mmol/1, 7.8 mmol/l<OGTT 2-h BG <11.1 mmol/1; DM: fasting BG>7 mmol/1 and/or OGTT 2-h BG>11.1 mmol/1.
Metabolic syndrome according to the International Diabetes Federation definition (IDF-MS): waist circumference over 90 cmin men and over 80 cmin women, and the presence of any two altered factors : (i) BP: SBP >130 mmHg and/or DBP >85 mmHg and/or subjects who received anti hypertension drug therapy; (ii) triglycerides: >1.7 mmol/1; (iii) HDL-C: <0.9 mmol/1, in men and < 1.1 mmol/1, in women; (iv) plasmatic glucose: >5.6 mmol/1, and/ or subjects who received anti-diabetic drug therapy.
Analysis of data
All analyses were conducted by using the Statistical Package for Social Sciences, version 13.0 (SPSS Inc, Chicago, IL). Continuous variables were described by using mean ± standard deviation (SD). Differences between the baseline measurements and the study end-point data were described by using means and 95% confidence intervals. Categorical variables were described by using percentages. The unpaired t-test, paired t-test and χ2 analysis were used. For inter-group data comparison, the unpaired t-test was used to compare all continuous variables and χ2 analysis was used for categorical variables. For comparison of the baseline measurements and the study end-point data of the same group, a paired t-test was used to compare all continuous variables. Except the drug dosage, the other parameters were normally distributed. A p-value <0.05 was considered statistically significant.
Generalized linear model (GLM) repeated-measures analysis was used to compare the clinical and laboratory data of the 29 patients on fosinopril+indapamide combination therapy with respect to the baseline values, those at the 14-month end-point and discontinuation of indapamide, and those at the 6-month end-point of continuous use of fosinopril alone.
A stepwise multiple linear regression analysis was used to evaluate potentially confounding factors related to the difference of AUCG between the baseline value and the end-point value. The dependent variables were the difference of AUCG between the baseline value and the end-point value, and the independent variables were sex, age, the baseline value of fast BG, 2-h postprandial BG, BW, BMI, WC and WHR, the difference of BW, BMI and WC before and after treatment. The stepwise model criteria: probability of F to enter <0.050, probability of F to remove >0.100.
In the F/I group, the relationship between the daily fosinopril dosage and the K difference before and after treatment was assessed using the Spearman correlation.
Results
Patient characteristics
Baseline characteristics of the patients enrolled in the two groups did not differ significantly in sex, age, IGT, MS, BW, BMI, WC, WHR, BP and HR (). During the follow-up period, 22 patients in the F/I group developed a cough, and 14 of them withdrew from the study because of the complaint; two patients withdrew from the study because of thirst, polydipsia and laboratory BG consistent with the diagnosis of DM; and another one withdrew from the study because BG at 5 months for OGTT test was consistent with the diagnosis of DM. In the F group, 24 patients developed a cough, and 18 of them withdrew from the study. There was no significant difference in the number of patients who withdrew from the study because of cough between the two groups (p=0.528). A total of 89 patients (45 in the F/I group and 44 in the F group) completed a mean of 14-month follow-up ranging from 12 to 16 months. The baseline characteristics of the two group patients did not differ significantly in sex, age, IGF, MS, BW, BMI, WC, WHR, BP and HR ().
Table II. Baseline characteristics of patients who completed 12–16 months follow-ups.
BP and HR change
In 12–16 months treatment, patients of the F/I group received 5–20 mg oral fosinopril daily (geometric means 8.2 mg) and indapamide 1.25–2.5 mg (geometric means 2.2 mg). Fosinopril+indapamide combination significantly reduced SBP/DBP. Patients of the F group received 5–20 mg oral fosinopril daily (geometric means 10.1 mg). Fosinopril alone significantly reduced SBP/DBP (). The BP control rate (SBP/DBP< 140/90 mmHg) was 93.3% (42/45) in the F/I group, and 88.6% (39/44) in the F group. There was no significant difference between the two groups (p=0.439).
Table III. Treatment outcomes of patients who completed the follow-ups (mean±SD).
Changes in BW, BMI, WC and WHR
BW, BMI and WC decreased significantly in patients treated with fosinopril alone, and remained insignificantly changed in patients receiving fosinopril+ indapamide combination therapy. There was a significant difference in BW and BMI difference before and after treatment between the two groups ().
Table IV. Difference between baseline and end-point of treatment outcomes [means (95% confidence interval)].
Changes in BG and ISI
Both fast, post OGTT and AUCG decreased significantly, and ISI (composite) increased significantly in patients receiving fosinopril alone, and vice versa in patients receiving fosinopril+indapamide combination therapy ( and ).
A stepwise multiple linear regression analysis showed that in the F group, three variables (the baseline value of 2-h postprandial BG, the difference of BW and WC before and after treatment) were related to the difference of AUCG in the final regression model (R2=0.329, p<0.001); in the F/I group, two variables (the baseline value of BMI and the difference of WC before and after treatment) were related to the difference of AUCG in the final regression model (R2 = 0.244, p=0.003).
BG was re-tested in 92 patients (44 in the F group and 48 in the F/I group) at the 14-month end-point because the other 32 patients withdrew from the follow-up. The occurrence of DM was 14.6% (7/48) in the F/I group, and 0% (0/44) in the F group (p=0.014). Conversion from NGT to IGT or DM was 20.7% (6/29) in the F/I group and 3.4% (1/29) in the F group (p=0.073); conversion from IGT to DM was 31.6% (6/19) in the F/I group, and 0% (0/15) in the F group (p=0.040).
Of 29 patients who were converted from fosinopril+indapamide combination therapy to fosinopril alone, BG decreased significantly ().
Table V. Clinical and laboratory data changes in 29 patients who converted from fosinopril+indapamide combination therapy to fosinopril alone.
All seven patients whose BG rose to the level consistent with the diagnosis of DM were MS patients, of whom one patient was lost to follow-up, and the remaining six patients were neither informed of the BG level nor put on diet control and BG treatment. After discontinuation of indapamide, they continued with fosinopril alone and were followed up for another 4–12 months, during which two patients were converted from DM to NGT, two converted from DM to IGT and two remained DM.
K and UA change
UA decreased significantly and K remained unchanged in patients receiving fosinopril alone and vice versa in the F/I group (). In the F/I group, there was no significant correlation between the daily fosinopril dosage and the K difference before and after treatment (r=–0.166, p=0.275).
In 29 patients who changed from fosinopril+ indapamide combination therapy to fosinopril alone, UA decreased significantly and K increased within the normal range ().
Discussion
The results of the present study suggest that fosinopril alone was able to improve GT, but fosinopril+ indapamide combination therapy impaired GT in Chinese hypertensive patients. Fosinopril alone was able to reverse fosinopril+indapamide-induced GT impairment in part of these patients.
ACEI can reduce the development of new DM onset in hypertensive patients, though there are controversial reports about the effect of ACEI on GT in hypertensive patients. Gress et al. (Citation18) reported that in the treatment of mild hypertension, enalapril did not significantly influence fasting BG in a 4-year follow-up. However, Kinoshita et al. (Citation19) reported that treatment with temocapril for 16 weeks improved insulin sensitivity and reduced fasting BG in 36 hypertensive patients. Wu et al. (Citation20) reported that fasting BG decreased significantly in 15 Chinese hypertensive patients who were treated with benazepril for uremia for 10 weeks. Our study found that treatment with fosinopril alone for 14 months significantly decreased both fasting and postprandial BG, improved insulin sensitivity and lowered UA level in hypertensive patients.
Previous studies (Citation21,Citation22) have demonstrated that beta-adrenergic receptor antagonists may significantly increase BW of hypertensive patients, but there are few studies reporting the influence of ACEI treatment on BW and WC of hypertensive patients. The results of the present study showed that BW decreased by a mean of 1.5 kg and WC decreased by 1.3 cm in patients receiving fosinopril alone. Previous studies (Citation23,Citation24) have demonstrated that BG is correlated with BW and WC. The results of the present study showed that BG reduction in patients who were administered with fosinopril alone was significantly correlated with BW and WC reduction, suggesting that the effect of ACEI in improving GT is related to ACEI-induced BW and WC reduction in Chinese hypertensive patients, the mechanism of which needs to be further observed in future studies.
There are controversial reports about the effect of indapamide on BG. Weidmann (Citation13) reported that oral administration of indapamide 1.25 mg/day for 2–12 months did not alter BG significantly in hypertensive patients. Leonetti et al. (Citation14) reported that treatment with three doses of indapamide (1.5 mg/ day, 2 mg/day and 2.5 mg/day) for 2 years did not change the BG of hypertensive patients significantly. However, Osei et al. (Citation15) reported that treatment with oral administration of indapamide (2.5 mg/day) for 24 weeks raised both fasting and postprandial BG levels in 13 patients with hypertension and diabetes. There are few studies reporting the effect of ACEI+indapamide combination therapy on BG. Asmar et al. (Citation25) reported that no significant change in BG was observed in hypertensive patients treated with combination of indapamide (0.625 mg/day) and perindopril (2 mg/day) for 12 months. Elisaf et al. (Citation26) reported that fasting BG increased significantly in 14 hypertensive patients treated with combination of indapamide (2.5 mg/day) and perindopril (4 mg/ day) for 4 weeks compared with that before treatment. The present study found that fasting and postprandial BG increased significantly in hypertensive patients treated with fosinopril+indapamide combination therapy for 14 months, and in seven patients the rise even reached the diagnostic criteria for DM, in whom K decreased and UA increased. The present study showed that fosinopril alone improved GT, seemingly concluding that fosinopril+indapamide-induced GT impairment in hypertensive patients was related to indapamide, and that prolonged use of indapamide may cause severe impairment to GT in hypertensive patients. The results of the present study and the above-mentioned studies suggest that there are different results about the effect of indapamide on BG. Apart from the dosage and follow-up duration, other factors contributing to these differences remain unclear. Leonetti et al. (Citation14) reported that the decreased incidence of hypokalemia induced by indapamide was related to different regions of Italy, the highest in northern Italy (17%), intermediate in the central region (14%) and the lowest in southern Italy (2%). Therefore, it is considered that influences of indapamide treatment on BG may be related to races. Chinese hypertensive patients may be more sensitive to the influence of indapamide on BG, but further study is needed to prove it. The results of the present study showed that elevated BG induced by fosinopril+indapamide combination therapy was significantly correlated with BMI of the hypertensive patients, suggesting that fosinopril+indapamide combination therapy should be used with caution in overweight or obese hypertensive patients to prevent BG from further increasing.
There are studies (Citation10,Citation16) suggesting that a high dose or prolonged use of diuretics impairs GT, mainly through chronic hypokalemia. Hypokalemia is the main action mechanism of diuretics in impairing GT (Citation27). The present study showed that fosinopril alone did not influence K level significantly, fosinopril+ indapamide combination therapy caused K to decrease significantly, and different oral doses of fosinopril did not seem to be related to K change significantly, suggesting that the reason why ACEI cannot prevent indapamide-induced GT impairment may be related to the fact that ACEI cannot prevent indapamide-induced potassium deficits in the body.
The present study showed that when the 29 patients were converted from fosinopril+indapamide combination therapy to fosinopril alone, fasting and postprandial BG decreased significantly. In the six patients whose BG reached the diagnostic criteria of DM after fosinopril+indapamide combination therapy and changed to fosinopril alone, four patients converted to NGT or IGT, and the other two remained diabetic, suggesting that GT impairment induced by one antihypertensive protocol may be reversed by alteration to another antihypertensive protocol. The present study also suggests that clinically, when BG is elevated or reaches the level consistent with the diagnosis of DM in patients who are receiving indapamide treatment, it is not necessary to initiate antihyperglycemic therapy immediately; rather, ACEI could be used instead of indapamide to observe BG change.
Dry cough is the most often reported and troublesome complication associated with ACEI use. The incidence of ACEI-induced cough varies in published reports,ranging from 5% to 35% (Citation28–30). Dry cough occurred in 37.1% (46/124) patients in our series, and 25.8% (32/124) patients withdrew from the study because of the complaint. The reason that the incidence of cough in our study was higher than that of other studies is not clear. There are studies reporting that the cough occurrence of using imidapril and benazepril for 6 weeks was 14.5% (41/282) and 24.6(70/284) in Chinese patients (Citation31), while that for 12 weeks was 0.9% (1/108) and 7.0 (8/115) in Japanese patients (Citation32). Dicpinigaitis (Citation33) reported that the incidence of ACEI-induced cough was high in Chinese patients, suggesting that the occurrence of cough may be related to racial differences.
Acknowledgments
The study was supported by the Major State Basic Research Development Program of the People's Republic of China (No.G2000156905) and Shanghai Medical Development Foundation (No. 2000I-ZD001). We thank Mr. Le-zhi Zhang for technical help, Mr. Luo-man Zhang for statistical analysis support, and our clinical colleagues, especially Hong-juan Mao, Li Gong, Lin Chen, Shao-Ping Cheng and Hong Wu for their constructive suggestions.
Declaration of interest: The authors declared no conflict of interest.
References
- Neal B, MacMahon S, Chapman N. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: Results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–1964.
- Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, . ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, . Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Mansia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . European Society of Hypertension; European Society of Cardiology. 2007 ESH-ESC Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press. 2007;16:135–232.
- Dunder K, Lind L, Zethelius B, Berglund L, Lithell H. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: Population based cohort study. BMJ. 2003;326:681–686.
- Verdecchia P, Reboldi G, Angeli F, Borgioni C, Gattobigio R, Filippucci L, . Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43:963–969.
- Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28:2261–2266.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med. 2004;351:585–592.
- Labeeuw M, Pozet N, Zech PY, Hadj-Aissa A, Finaz de Villaine J, Laville M. Influence of acute administration of ramipril on the excretion of uric acid. Arch Mal Coeur Vaiss. 1987;80:870–874.
- Shargorodsky M, Boaz M, Davidovitz I, Asherov J, Gavish D, Zimlichman R. Treatment of hypertension with thiazides: Benefit or damage-effect of low- and high-dose thiazide diuretics on arterial elasticity and metabolic parameters in hypertensive patients with and without glucose intolerance. J Cardiometab Syndr. 2007;2:16–23.
- Saruta T, Ogihara T, Matsuoka H, Suzuki H, Toki M, Hirayama Y, . Antihypertensive efficacy and safety of fixed-dose combination therapy with losartan plus hydrochlo-rothiazide in Japanese patients with essential hypertension. Hypertens Res. 2007;30:729–739.
- Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: A quantitative review. Hyhpertension. 2006;48:219–224.
- Weidmann P. Metabolic profile of indapamide sustained-release in patients with hypertension: Data from three randomised double-blind studies. Drug Saf. 2001;24:1155–1165.
- Leonetti G, Rappelli A, Salvetti A, Scapellato L. Long-term effects of indapamide: Final results of a two-year Italian multicenter study in systemic hypertension. Am J Cardiol. 1990;65:67H–71H.
- Osei K, Holland G, Falko JM. Indapamide: Effects on apoprotein, lipoprotein, and glucoregulation in ambulatory diabetic patients. Arch. Intern. Med. 1986;146:1973–1977.
- Gorden P. Glucose intolerance with hypokalemia. Failure of short-term potassium depletion in normal subjects to reproduce the glucose and insulin abnormalities of clinical hypokalemia. Diabetes. 1973;22:544–551.
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470.
- Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–912.
- Kinoshita M, Nakaya Y, Harada N, Takahashi A, Nomura M, Bando S. Combination therapy of exercise and angiotensin-converting enzyme inhibitor markedly improves insulin sensitivities in hypertensive patients with insulin resistance. Circ J. 2002;66:655–658.
- Wu Z, Bao X. Effects of benazepril on insulin resistance and glucose tolerance in uremia. Clin Nephrol. 1998;50:108–112.
- Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: Beta-adrenergic receptor blockers and weight gain: A systematic analysis. Hypertension. 2001;37:250–254.
- Messerli FH, Bell DS, Fonseca V, Katholi RE, McGill JB, Phillips RA, .; GEMINI Investigators. Body weight changes with beta-blocker use: Results from GEMINI. Am J Med. 2007;120:610–615.
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–9.
- Despres JP, Lemieux I. Abdominal obesity and the metabolic syndrome. Nature. 2006;444:881–887.
- Asmar RG, London GM, O’Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: A comparison with atenolol. Hypertension. 2001;38:922–926.
- Elisaf MS, Theodorou J, Pappas H, Papagalanis N, Katopodis K, Kalaitzidis R, . Effectiveness and metabolic effects of perindopril and diuretics combination in primary hypertension. J Hum Hypertens. 1999;13:787–791.
- Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism. 1980;29:498–502.
- Sebastian JL, McKinney WP, Kaufman J, Young MJ. Angiotensin-converting enzyme inhibitors and cough: Prevalence in an outpatient medical clinic population. Chest. 1991;99:36–39.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. Ann Intern Med. 1992;117:234–242.
- Simon SR, Black HR, Moser M, Berland WE. Cough and ACE inhibitors. Arch Intern Med. 1992;152:1698–1700.
- Imidapril and Benazepril Clinical Study Cooperation Unit. Comparative study of hypotensive efficacy and the cough occurrence of imidapril versus benazepril. Chin J Cardiol. 2004;4:304–307.
- Saruta T, Omae T, Kuramochi M, Iimura O, Yoshinaga K, Abe K, . Imidapril hydrochloride in essential hypertension: A double-blind comparative study using enalapril maleate as a control. J Hypertens Suppl. 1995;13:523–530.
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S.