Abstract
Objective. Regression of left ventricular (LV) hypertrophy and albuminuria in hypertension has previously been shown to reduce clinical cardiovascular events and death. We aimed to investigate the associations of regression of electrocardiographic (ECG) LV hypertrophy and albuminuria with the incidence of revascularization. Methods. In 9193 hypertensive patients included in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, we measured urine albumin/creatinine ratio (UACR), LV hypertrophy by electrocardiography, serum high-density lipoprotein (HDL) cholesterol, and blood pressure after 2 weeks of placebo treatment and yearly during 5 years of anti-hypertensive treatment with either an atenolol- or a losartan-based regimen. The incidence of coronary and peripheral revascularization was recorded. Results. In Cox regression analyses adjusted for treatment allocation and continent, high time-varying Sokolow–Lyon voltage (hazard ratio [HR]=1.01 [1.00–1.02], p=0.01), but not time-varying Cornell product or UACR, predicted coronary revascularization together with low time-varying HDL-cholesterol, low time-varying pulse pressure, high Framingham risk score and history of angina pectoris. Adjusted for treatment allocation and continent, high time-varying Sokolow–Lyon voltage (HR=1.01 [1.00–1.03], p=0.02), but not time-varying Cornell product or UACR, predicted peripheral revascularization together with high time-varying pulse pressure, high Framingham risk score, history of peripheral vascular disease and prior myocardial infarction. Conclusion. Higher Sokolow–Lyon voltage during antihypertensive treatment, but not UACR or the Cornell voltage–duration product, was independently associated with higher incidence of coronary as well as peripheral revascularization.
Introduction
Left ventricular hypertrophy (LVH) is a strong predictor of cardiovascular (CV) morbidity and mortality (Citation1–3). The underlying pathophysiological mechanisms are not fully understood, but may involve systemic inflammation and atherosclerosis as well as direct myocardial alterations. The resulting increase in LV mass is associated with increased myocardial oxygen demand and reduced tolerance of myocardial ischaemia (Citation4). This creates a vicious circle, in which adaptive changes in the hypertrophied myocardium further increase the susceptibility for ischaemic episodes. A recent study showed that concentric LVH was independently associated with coronary artery calcium, supporting that LVH is a risk factor for subclinical atherosclerosis (Citation5). LVH is also associated with atherosclerosis in other vascular territories (Citation6). Regression of LVH is found to reduce the risk of CV events and sudden cardiac death in a hypertensive population (Citation7,Citation8). Likewise, one may speculate whether regression of LVH may represent lesser progression of atherosclerosis, or maybe even reductions in established atherosclerotic disease.
Urine albumin/creatinine ratio (UACR) is associated with LVH in hypertensive (Citation9) and healthy persons (Citation10). Increased UACR represents endothelial damage at the glomerulus, reflecting systemic vascular dysfunction. It has been related to atherosclerosis in healthy subjects (Citation11) as well as in hypertensive patients (Citation12). Cottone et al. demonstrated that increased UACR was associated with endothelial activation in patients with uncomplicated essential hypertension with no clinical evidence of atherosclerosis (Citation13). This suggests that activation of the endothelium may precede the development of atherosclerosis.
The Losartan Intervention for Endpoint reduction in hypertension (LIFE) study is a prospective study of hypertensive patients with electrocardiographic LVH randomized to treatment with losartan or atenolol. We have previously demonstrated in this population that LVH defined by Sokolow–Lyon voltage criteria predicted both coronary and peripheral revascularization (Citation14). However, to the best of our knowledge, no studies have investigated whether reduction of LVH or UACR per se reduces the rates of revascularization. Therefore, the present study was undertaken to investigate: (i) the effect of regression of LVH and UACR on the incidences of coronary and peripheral revascularization in hypertensive patients and (ii) whether regression of LVH after completed revascularization reduced CV mortality.
Materials and Methods
Patient population
A total of 9193 hypertensive patients aged 55–80 years with previously untreated or treated essential hypertension and electrocardiographic LVH were included in the prospective, double-blind LIFE study. As described in detail elsewhere (Citation15), patients were randomized to a losartan- or atenolol-based regimen and treated to a target blood pressure of <140/90 mmHg. In all patients, UACR, LVH by electrocardiography, serum high-density lipoprotein (HDL)-cholesterol, plasma glucose and blood pressure were measured after 2 weeks of placebo treatment and yearly during the mean 4.8 years of anti-hypertensive treatment. Patients with angina pectoris requiring treatment with a beta-blocker or a calcium antagonist were excluded.
The revascularization analyses were a pre-specified part of the LIFE protocol with revascularization being a pre-specified secondary endpoint (Citation15). Coronary revascularization included all coronary artery revascularization procedures (angioplasty, atherectomy and stent) and heart transplant (n< 5). Peripheral revascularization included all non-coronary artery vascular surgeries and revascularization procedures (aortic aneurysm repair, carotid and peripheral revascularizations and amputations because of arterial vascular insufficiency and diabetes mellitus). Each revascularization event was reported by the investigators and verified by an independent endpoint classification committee consisting of two cardiology experts and based on definitions in a pre-defined endpoint manual. All patients gave written informed consent, and the protocol was approved by regional ethical committees.
Electrocardiography
Electrocardiograms (ECGs) were taken at baseline, at 6 months and at yearly follow-up intervals. ECGs were interpreted by observers blinded to clinical data at the Core Laboratory at Sahlgrenska University Hospital/Östra in Gothenburg, Sweden. LVH was defined as the product of QRS duration multiplied by the Cornell voltage combination (RaVL+SV3, with 6 mm added in women) higher than 2440 mm×ms or Sokolow–Lyon voltage (SV1+RV5/6) higher than 38 mm.
Renal evaluation
Albuminuria was measured as the albumin/creatinine ratio by standard methods using a turbidimetric method (Hitachi 717 Analyzer; Boehringer Mannheim, Mannheim, Germany) in a single spot urine collection on the morning of the baseline ECG and after 12 months. Both serum and urine creatinine were analysed using the Jaffè reaction without deproteinizing and then quantified by a photometric method (Hitachi 717 Analyzer). The urine albumin concentration was expressed as a ratio to urinary creatinine concentration, to provide a composite measure of renal glomerular capillary permeability adjusting for urine dilution.
Statistical analysis
Statistical analyses were performed using SPSS 12.0 (SPSS, Chicago, IL, USA) software. Continuous variables are presented as means and were compared using one-way analysis of variance (ANOVA) and Student's t-test. Categorical variables are presented as proportions and were compared using Pearson's chi-square test. Based on the distribution of residuals from the models and test for linearity and proportional hazard assumption in Cox regression analyses only UACR required logarithmic transformation. Cox regression analysis with time-varying covariates was used in order to evaluate the importance of baseline as well as in-treatment values through year five of treatment. Multiple Cox regression analyses were used to adjust for randomized treatment, continent (USA vs European countries), Framingham risk score (including age, gender, total- and HDL-cholesterol, systolic blood pressure, smoking, diabetes and LVH) (Citation16) and different known CV diseases (previous angina pectoris, myocardial infarction, cerebral vascular disease (stroke and transient ischemic attack) and peripheral vascular disease) as well as in-treatment pulse pressure and in-treatment HDL-cholesterol. Backward selection was used until all variables but randomized treatment had p < 0.1, and only these significant variables were included in the final models. In-treatment Sokolow–Lyon voltage and Cornell product were used both as continuous as well as dichotomous variables and did not interact with age or gender. Additionally, data were analysed using baseline and 1-year ECGs to predict revascularization after one year of treatment in (excluding revascularization during the first year to avoid bias). In the time-varying analyses, however, all revascularization events were included. In Pearson's chi-square analyses of CV mortality after revascularization, patients were divided according to LVH presence on baseline ECG (). Two-tailed p< 0.05 gave statistical significance.
Results
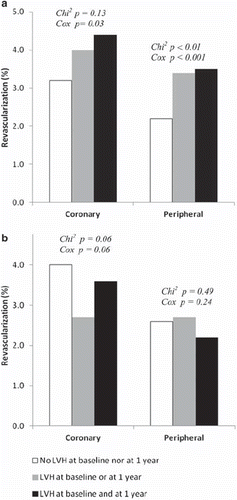
During the mean 4.8 years of follow-up, 337 patients (3.7%) underwent coronary revascularization and 231 patients (2.5%) underwent peripheral revascularization. Background variables for these patients have been presented previously (Citation14). We divided the LIFE population into three groups based on LVH by Sokolow–Lyon voltage criteria (). The intermediate group (LVH at baseline or at 1 year) was included to differentiate clearly between the other two groups. Because of measurement variation, regression to the mean and treatment, some patients were moving back and forth between LVH groups during the first year of the study. Patients with LVH defined by Sokolow–Lyon voltage at baseline and 1 year had significantly higher systolic blood pressure, pulse pressure, creatinine and Framingham risk score compared with patients without LVH at either time. However, patients with LVH by this index also had less abnormal metabolic risk factors [higher HDL-cholesterol and lower body mass index (BMI), total cholesterol and serum glucose].
Table I. Baseline characteristics in patients according to LVH status by Sokolow–Lyon voltage criteria.
Patients with LVH at baseline and/or year-1 defined by Sokolow–Lyon voltage showed trends to more frequent revascularization, as illustrated by . Peripheral revascularization was significantly different between groups (p=0.01). Coronary revascularization tended to occur more frequently in patients with increased LVH by Sokolow–Lyon voltage, although this trend did not attain statistical significance (p=0.13). In contrast, there was a trend of less frequent revascularization in patients with LVH at baseline and/or year-1 defined by Cornell voltage–duration product ().
In Cox regression analyses adjusted for treatment allocation and continent, coronary revascularization was predicted by high time-varying Sokolow–Lyon voltage and low time-varying pulse pressure, together with low time-varying HDL-cholesterol, high Framingham risk score and history of angina pectoris (). Time-varying Cornell product and time-varying UACR did not reach statistical significance. Peripheral revascularization was, after adjustment for treatment allocation and continent, predicted by high time-varying Sokolow–Lyon voltage, but not time-varying UACR or Cornell product, together with high time-varying pulse pressure, high Framingham risk score, history of peripheral vascular disease and prior myocardial infarction ().
Table II. Multivariate Cox regression models predicting revascularization in the whole group.
In the right column of , the same multivariate analyses are presented using LVH defined by Sokolow–Lyon voltage as a dichotomous variable. LVH by Sokolow–Lyon voltage was a borderline significant predictor of coronary revascularization together with low time-varying HDL-cholesterol, high Framingham risk score and history of angina pectoris. Regarding peripheral revascularization, LVH by Sokolow–Lyon voltage was not a significant predictor in multivariate analysis ().
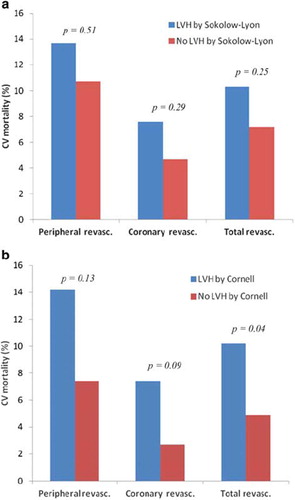
Of the 568 patients in the LIFE study who underwent revascularization, 46 patients died of CV disease within the study period. (a) and (b) show the relations between CV mortality after revascularization and LVH at baseline by Sokolow–Lyon voltage and Cornell voltage–duration product, respectively. Using Sokolow–Lyon voltage criteria, there was no significant association between LVH status and CV mortality after coronary or peripheral revascularization. However, LVH by Cornell product was associated with higher CV mortality after coronary or peripheral revascularization (p=0.04). In Cox regression analyses that adjusted for treatment allocation and continent, LVH by Cornell voltage–duration product, but not LVH by Sokolow–Lyon voltage predicted CV mortality, together with Framingham risk score and prevalent cerebral vascular disease ().
Table III. Multivariate Cox regression model predicting cardiovascular mortality after revascularization.
Discussion
The present study demonstrates that lack of regression of LVH, but not UACR, during anti-hypertensive treatment is independently associated with increased incidence of coronary and peripheral revascularization in a large hypertensive population. We can only speculate about the causal relationships, but the associations between revascularization and time-varying Sokolow–Lyon voltage were independent of time-varying pulse pressure indicating that the associations were not just related to less blood pressure reduction in patients with lack of LVH regression. A likely possibility could be that patients with persisting LVH are more likely to experience angina because of more myocardial tissue per vessel leading to angiography and in some cases coronary revascularization. Further, it may be likely that patients with persistent high LVH in parallel have more persistent peripheral structural vascular hypertrophy leading to more peripheral revascularization.
It was surprising that regression of UACR had no effect on coronary revascularization. Several data link albuminuria to CV complications and a few studies have specifically examined its relationship to atherosclerosis, concluding that albuminuria is directly correlated with angiographic evidence of coronary artery disease (Citation17,Citation18). The lack of association between UACR and coronary revascularization in our study might reflect that patients without reduction in UACR have more generalized atherosclerotic disease and are therefore not candidates for revascularization. Yet, we cannot exclude the possibility of a potential statistical type II error, even though our material includes over 500 events. Few studies have addressed the risk of peripheral artery disease in patients with microalbuminuria. The PREVEND study showed that microalbuminuria was independently associated with coronary heart disease, but not peripheral artery disease in the general population (Citation19). In contrast, C-reactive protein (CRP) was related to damage in all vascular beds, suggesting that different risk factors may play different roles in various types of vasculature.
In the present study, we found that persistently high Sokolow–Lyon voltage predicted coronary and peripheral revascularization, whereas time-varying Cornell voltage–duration product did not. This may be related to different patient characteristics at baseline according to LVH classification by Sokolow–Lyon voltage or Cornell product. Patients with LVH defined by Sokolow–Lyon voltage were younger and leaner and had higher pulse pressure and HDL-cholesterol compared with patients without LVH by this method (Citation20). In addition, they were more likely to be men and to smoke, both of which would tend to lead to greater clinical suspicion of peripheral arterial disease. Patients with LVH defined by Cornell product were older and had higher BMI and total cholesterol and a trend toward higher serum glucose compared with patients without LVH by this method. Consequently, one may speculate that patients recruited by the Cornell criteria were more likely to develop metabolic disorders with increased risk for surgical complications, possibly making them less suitable for surgery. Patients with Sokolow–Lyon with less co-morbidity may on the other hand be more likely to be offered revascularization.
One of the most important determinants of LV mass is body weight (Citation21), as increased body weight may increase heart size because of the need for higher cardiac output. Weight loss has been associated with a decrease in LV mass among hypertensives in several studies (Citation22,Citation23). During the mean 4.8 years of follow-up in the LIFE study, the study population experienced a small increase in weight (unpublished data). Consequently, our findings of LVH regression cannot be explained by changes in body weight.
Generally, the clinical judgments behind revascularization procedures are based on three factors: patient symptoms, patients’ co-morbidities and lastly, local medical traditions. As expected, we found that the frequency of both coronary and peripheral revascularization was higher in USA independently of traditional CV risk factors. To account for the latter, we adjusted our analyses for continent (USA vs European countries). If there hypothetically indeed were done revascularization procedures based on non-medical decisions in USA, this would only possibly dilute our results, and not strengthen the associations.
We have previously demonstrated that the incidence of peripheral revascularization in patients without peripheral vascular disease or albuminuria at baseline was lower in patients randomized to losartan vs atenolol-based antihypertensive treatment (Citation14). The mechanism for this beneficial effect of losartan was not clear. Studies of hypercholesterolaemic monkeys have shown that losartan was associated with regression of atherosclerotic lesions in the aorta, independent of effects on blood pressure (Citation24,Citation25). However, there is yet no direct evidence that inhibition of the renin–angiotensin–aldosterone system (RAAS) slows or reverses the progression of atherosclerosis in humans. Theoretically, in our population, atenolol might improve angina pectoris and worsen peripheral artery disease symptoms compared with losartan. As the present study was undertaken to evaluate the effect of regression of LVH and UACR per se, independent of medication effect, we have adjusted the analyses for study drug treatment.
After revascularization (either coronary or peripheral), we found that continuing LVH by Cornell voltage–duration product was a significant predictor of CV mortality. This would suggest, if persistently complete coronary revascularization had been produced by the interventions, that LVH assessed by Cornell product is associated with increased CV mortality by other mechanisms than the coronary atherosclerosis known to be associated with LVH. Whether this is related to LVH by itself or to the association of LVH with systemic atherosclerosis (Citation6) or other associated risk factors is unclear. LVH by Sokolow–Lyon criteria did not reach statistical significance, potentially related to loss of power because of the lower prevalence of LVH by this criterion than by Cornell product criteria, or other differences in risk profile.
Several studies have shown that patients with LVH have increased in-hospital mortality after coronary artery bypass graft (CABG) surgery (Citation26,Citation27). Recently, the first data assessing long-term mortality risk postoperatively were published, revealing that ECG LVH was a risk factor for all-cause late mortality, especially after the third postoperative year (Citation28). Lauer et al. followed 8166 patients for a mean 6 years after CABG surgery and evaluated quantitative ECG measures. One of the independent predictors of long-term all-cause mortality was Sokolow–Lyon voltage in the first or fourth quartile (Citation29). Cornell voltage showed a continuous risk pattern with increasing risk at increasing values. From these data together with our study, we can conclude that LVH regression should be an independent goal in the future therapy and follow-up after revascularization procedures in order to reduce the CV risk for these patients.
Limitations
Some limitations of our study must be taken into account. UACR was calculated based on a single spot urine collection. However, other studies have shown a close correlation between this ratio and measures of albuminuria from 24-h collection (Citation30). Patients with angina pectoris requiring treatment with a beta-blocker or a calcium antagonist, heart failure, known left ventricular ejection fraction <40% or recent myocardial infarction within the past 6 months were excluded from the LIFE study; hence our results may not be representative of the typical hypertensive population undergoing revascularization. Finally, our analyses cannot exclude the effect of potential, yet unknown, confounders that may cause microalbuminuria or LVH and require revascularization. As the LIFE study was not designed to investigate causal relationships between baseline predictors and revascularization, we can only draw correlational conclusions.
Conclusions
Continuing high Sokolow–Lyon voltage was, in contrast to UACR, associated with higher incidence of coronary as well as peripheral revascularization independent of prior CV disease and traditional CV risk factors including time-varying pulse pressure and HDL-cholesterol. After revascularization, LVH by Cornell voltage–duration product predicted CV mortality together with Framingham risk score and cerebral vascular disease.
Acknowledgements
This work was supported by Merck & Co., Inc. Drs Wachtell, Okin, Dahlöf, Devereux, Kjeldsen and Olsen have received grant support from Merck & Co, Inc., the sponsor of the LIFE study.
Declaration of interest: The authors alone are responsible for the content and writing of the paper.
References
- Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, . Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54.
- Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg. 2003; 75:S678–S684.
- Mehta SK, Rame JE, Khera A, Murphy SA, Canham RM, Peshock RM, . Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation. Hypertension. 2007;49: 1385–1391.
- Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90.
- Wachtell K, Okin PM, Olsen MH, Dahlöf B, Devereux RB, Ibsen H, . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: The LIFE Study. Circulation. 2007;116:700–705.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349.
- Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlöf B, . Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J. 2002;143:319–326.
- Lieb W, Mayer B, Stritzke J, Doering A, Hense HW, Loewel H, . Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: The MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant. 2006;21:2780–2787.
- Furtner M, Kiechl S, Mair A, Seppi K, Weger S, Oberhollenzer F, . Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J. 2005;26:279–287.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens. 1995;9:827–833.
- Cottone S, Mulé G, Nardi E, Lorito MC, Guarneri M, Arsena R, . Microalbuminuria and early endothelial activation in essential hypertension. J Hum Hypertens. 2007;21:167–172.
- Olsen MH, Wachtell K, Dahlöf B, Devereux RB, Ibsen H, Kjeldsen SE, . The effect of losartan compared with atenolol on the incidence of revascularization in patients with hypertension and electrocardiographic left ventricular hypertrophy. The LIFE study. J Hum Hypertens. 2006;20:460–464.
- Dahlöf B, Devereux R, de Faire U, Fyhrquist F, Hedner T, Ibsen H, . The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: Rationale, design, and methods. The LIFE Study Group. Am J Hypertens. 1997;10:705–713.
- Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362.
- Sukhija R, Aronow WS, Kakar P, Garza L, Sachdeva R, Sinha A, . Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98:279–281.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: An angiographic study. Am J Kidney Dis. 1999;34:918–925.
- Stuveling EM, Hillege HL, Bakker SJ, Asselbergs FW, de Jong PE, Gans RO, . C-reactive protein and microalbuminuria differ in their associations with various domains of vascular disease. Atherosclerosis. 2004;172:107–114.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlöf B. Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: The Losartan intervention for endpoint reduction (LIFE) in hypertension study. The Life Study Investigators. Hypertension. 2000;36:766–773.
- Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J Am Coll Cardiol. 1988;12:996–1004.
- Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH, Jr., Neaton JD, . Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91:698–706.
- Schillaci G, Pasqualini L, Vaudo G, Lupattelli G, Pirro M, Gemelli F, . Effect of body weight changes on 24-hour blood pressure and left ventricular mass in hypertension: A 4-year follow-up. Am J Hypertens. 2003;16:634–639.
- Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000; 101:1586–1593.
- Takai S, Jin D, Sakaguchi M, Muramatsu M, Miyazaki M. The regressive effect of an angiotensin II receptor blocker on formed fatty streaks in monkeys fed a high-cholesterol diet. J Hypertens. 2005;23:1879–1886.
- Lin CL, Hsu PY, Yang CT, Yang HY, Yang TY, Huang WH, . LV mass index significantly impacts on patient and renal outcomes in patients with coronary artery bypass grafting and poor left-ventricular function. Ren Fail. 2003;25:287–295.
- Christenson JT, Simonet F, Schmuziger M. The impact of arterial hypertension on the results of coronary artery bypass grafting. Thorac Cardiovasc Surgeon. 1996;44:126–31.
- Toumpoulis IK, Chamogeorgakis TP, Angouras DC, Swistel DG, Anagnostopoulos CE, Rokkas CK. The impact of left ventricular hypertrophy on early and long-term survival after coronary artery bypass grafting. Int J Cardiol. 2009;135: 36–42.
- Lauer MS, Martino D, Ishwaran H, Blackstone EH. Quantitative measures of electrocardiographic left ventricular mass, conduction, and repolarization, and long-term survival after coronary artery bypass grafting. Circulation. 2007;116:888–893.
- Eshøj O, Feldt-Rasmussen B, Larsen ML, Mogensen EF. Comparison of overnight, morning and 24-hour urine collections in the assessment of diabetic microalbuminuria. Diabet Med. 1987;4:531–533.