Abstract
Traditional cardiovascular risk factors have poor prognostic value for individuals and screening for subclinical organ damage has been recommended in hypertension in recent guidelines. The aim of this review was to investigate the clinical impact of the additive prognostic information provided by measuring subclinical organ damage. We have (i) reviewed recent studies linking markers of subclinical organ damage in the heart, blood vessels and kidney to cardiovascular risk; (ii) discussed the evidence for improvement in cardiovascular risk prediction using markers of subclinical organ damage; (iii) investigated which and how many markers to measure and (iv) finally discussed whether measuring subclinical organ damage provided benefits beyond risk prediction. In conclusion, more studies and if possible randomized studies are needed to investigate (i) the importance of markers of subclinical organ damage for risk discrimination, calibration and reclassification; and (ii) the economic costs and health benefits associated with measuring markers of subclinical organ damage.
Introduction
The traditional major cardiovascular risk factors, such as an blood pressure, cholesterol or smoking are present in as many as 85% of coronary heart disease cases (Citation1). However, 90% of young adults and middle-aged men and women in various cohorts have at least one elevated risk factor (Citation2) and the vast majority of these individuals will not experience an adverse cardiovascular event. This is the reason why these risk factors even in combination have poor screening performance for cardiovascular disease (Citation3). As a consequence, current guidelines for the prevention of cardiovascular disease such as the Third Report of the National Cholesterol Education Program (Citation4) and especially the Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and European Society of Cardiology (ESC) (Citation5,Citation6) have included measurements of subclinical organ damage in risk stratification. Markers of subclinical organ damage are believed to be precursors of established cardiovascular disease and not just etiological risk factors. Because of this closer association with disease, the markers are thought to improve the individual risk prediction beyond that of the traditional risk factors. Consequently, subjects with presence of subclinical organ damage are allocated to higher risk categories than based on the levels of the traditional risk factors in the respective risk charts.
In the following, we will review recent reports linking markers of subclinical organ damage in different target organs with cardiovascular disease and discuss the evidence linking subclinical organ damage to better risk stratification.
Risk associations in different target organs
Heart. Left ventricular hypertrophy (LVH) is highly prevalent in the hypertensive population and associated with increased cardiovascular risk independent of traditional cardiovascular risk factors, including office and ambulatory blood pressure (Citation7). The electrocardiogram (ECG) is a cheap and easy accessible tool for detecting LVH. There exist several ECG criteria for diagnosing LVH, but the two most recommended are the Sokolow–Lyon and Cornell voltage–duration product criteria (Citation5). A common drawback is low sensitivity compared with echocardiography (Citation8). However, echocardiography also has technical limitations, and more accurate determination of LVH can be achieved with nuclear magnetic resonance imaging (Citation9), although this technique is not yet accessible in the daily clinic. Other ECG characteristics have been associated with subclinical cardiac damage in hypertensive subjects. The voltage of the R wave in lead aVL (Citation10), left bundle branch block (Citation11), prolonged ventricular repolarization (Citation12) and a ST strain pattern (Citation13) were all associated with cardiovascular risk independent of traditional risk factors as well as LVH and may reflect cardiac fibrosis, sub-endocardial ischemia or systolic dysfunction. The NOVACODE estimate of LV mass index combines several of these ECG measurements and predicted cardiovascular risk slightly better than the Sokolow–Lyon and Cornell voltage–duration product criteria adjusted for the Framingham variables in a prospective study of 8000 individuals (Citation14).
In addition to LVH, echocardiography provides information on cardiac function. Especially, diastolic dysfunction assessed with tissue Doppler techniques is highly prevalent in the elderly hypertensive population without overt heart failure (Citation15) and independently associated with cardiovascular risk (Citation16). Finally, a combination of ECG and echocardiography has additive predictive value as demonstrated in studies with echo–LVH and ECG–LVH (Citation17) as well as ECG–strain (Citation18).
Blood vessels. Ultrasound of the carotid arteries is a relatively simple and non-invasive method to detect early atherosclerosis. Presence of carotid plaques and increased intima-media thickness (IMT) correlates with increased cardiovascular risk independent of traditional risk factors (Citation19,Citation20). The two markers predict cardiovascular risk independently of each other (Citation21,Citation22), probably related to the fact that they reflect different biological aspects of atherogenesis: IMT reflects a hypertensive hypertrophic response of the medial cells and plaques a later stage of atherosclerosis related to inflammation (Citation23). Plaques can be further assessed qualitatively and quantitatively with echogenicity, heterogeneity, total plaque area and volume, which strengthen the risk associations (Citation24). Atherosclerosis is also reflected in blood vessels by increased arterial stiffness. The current golden standard to measure arterial stiffness is carotid–femoral pulse wave velocity (PWV) and increased PWV has been associated with increased cardiovascular risk in numerous population studies independent of other baseline risk factors (Citation25). Another indicator of generalized atherosclerosis is the ankle brachial index (ABI) and a low index (≤0.9) as well as a high index (>1.40) has been associated with increased risk independent of the Framingham risk score variables (Citation26). Finally, subclinical coronary atherosclerosis is reflected in increased coronary artery calcification, which can be detected by electron beam computer tomography. An increased coronary artery calcium score (CAC) has been positively associated with cardiovascular risk independent of traditional risk factors across several age and ethnic groups (Citation27).
Kidney. Renal damage indicated by reduced renal function and/or elevated urinary excretion of albumin is associated with an increased risk of cardiovascular events. Renal insufficiency is categorized according to glomerular filtration rate (GFR) (Citation28) and moderate (60>GFR≥30 ml/min/1.73 m2) (Citation29) as well as mild (90>GFR≥60 ml/min/1.73 m2) (Citation30) renal impairment have been associated with an independent cardiovascular risk. An elevated serum creatinine will only detect moderate to severe renal insufficiency (Citation31). Instead, GFR can be estimated using information on serum creatinine, age sex and race with the Modification of Diet in Renal Disease (MDRD) formula (Citation32) or by estimating the creatinine clearance rate with the Cockcroft–Gault formula using serum creatinine, age, weight and sex (Citation33). A recent study in high-risk hypertensive subjects suggested that the MDRD formula was superior to the Cockcroft–Gault formula in predicting cardiovascular events (Citation34). Furthermore, MDRD has been shown to be more accurate in subjects with low GFR (Citation35). Urine excretion of albumin can be detected with a simple dipstick test or with higher sensitivity using a single urine specimen and expressed as the urine albumin/creatinine ratio (UACR). Low estimated GFR and high urine albumin excretion were not highly associated in an Italian prospective study and each predicted cardiovascular risk independent of the other suggesting that the two markers of kidney function provide complementary information (Citation36). The association between cardiovascular risk and albumin excretion is continuous and even at UACR levels around 1 mg/mmol, there is a considerably increased cardiovascular risk (Citation37) suggesting presence of subclinical organ damage and challenging the traditional ranges of microalbuminuria which are UACR≥2.5 mg/mmol for men and ≥3.5 mg/mmol for women (Citation5). UACR in the range of microalbuminuria and below is believed to be not only a marker of renal dysfunction but also a marker of generalized endothelial dysfunction (Citation38).
shows commonly used thresholds for different measures of subclinical organ damage. For practical clinical purposes, dichotomized values are used, commonly based on the value of the top tertile or quartile of a population, although the risk associations are continuous below the arbitrarily chosen cut points.
Table I. Currently recommended thresholds suggesting subclinical organ damage and increased cardiovascular risk.
Risk prediction with subclinical organ damage
The benefit for risk stratification of screening for subclinical organ damage is primarily based on cohort studies. There has not been performed any large prospective studies in which subjects have been randomized to risk stratification with and without markers of subclinical organ damage. Until recently, many studies have assumed an incremental predictive value of a new marker by demonstrating a significant hazard ratio for the new marker adjusted for the traditional risk markers. In addition, additive predictive power has often been analyzed by examining increments in the area under the receiver operating curve (ROC). A ROC analysis plots sensitivity (fraction of true positive tests) against 1−specificity (fraction of false positive test) for a given combination of risk markers. The ROC is a measurement of how well a model can discriminate those who have the disease from those who do not. However, this is not sufficient to prove that the marker has clinical utility (Citation39) and from a clinical viewpoint it is equal important that a new marker results in improvement in model calibration, meaning that the predicted risk agrees better with the actual risk (Citation40). The resulting reclassification should also be clinical relevant and the new marker should reclassify a substantial proportion of originally intermediate-risk subjects as high-risk or vice versa. In addition, such reclassification should lead to changes in clinical management reducing the risk for cardiovascular disease. Few studies on markers of subclinical organ damage have incorporated all these features. In the meta-analysis from the Ankle Brachial Index Collaboration, inclusion of ABI using the Framingham risk score in 24955 men and 23339 women without a history of cardiovascular disease resulted in reclassification and modification of treatment recommendation in approximately 19% of men and 36% of women (Citation26). However, in men the main effect of ABI was changing high-risk estimates into intermediate risk and in women changing low-risk estimates into intermediate risk, none of which changed clinical management radically.
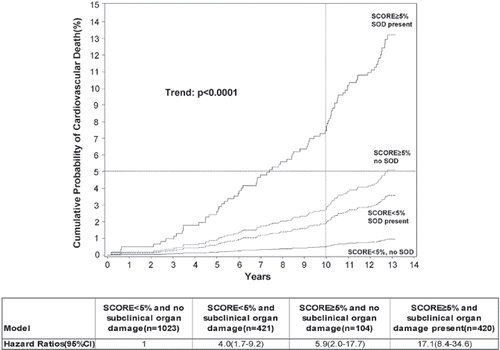
In a cohort study of approximately 2000 healthy individuals, aged 41–71 years who were not receiving any cardiovascular, anti-diabetic or lipid-lowering medications we measured traditional risk factors as well as PWV, LVMI, carotid atherosclerotic plaques and UACR. During a mean follow-up of 12.8 years, cardiovascular end points were recorded through national registries. We compared the risk stratification with ESH risk chart including the four markers of subclinical organ damage with Systemic Coronary Risk Evaluation (SCORE) (Citation41), a risk equation based on age, sex, systolic blood pressure, total cholesterol and smoking in 1344 subjects with higher than optimal blood pressure. The ESH risk chart allocated 368 (p<0.001) more subjects to higher-risk categories than SCORE. However, using the current guidelines on initiation of antihypertensive treatment, the two risk charts recommended treatment to the same subjects in 89% and produced similar sensitivities (79 vs 79%), specificities (46 vs 50%), positive (14 vs 15%) and negative (95 vs 96%) predictive values for a composite end point consisting of cardiovascular death and non-fatal myocardial infarction and stroke () (Citation42). We also examined whether markers of subclinical damage added incremental predictive value to SCORE (Citation43). As illustrated in , inclusion of markers of subclinical organ damage enabled further stratification of SCORE risk categories into subjects with very high or very low risk corresponding to SCORE≥5% and presence of subclinical organ damage or SCORE<5% and no damage, respectively. Although the markers predicted 10-year risk of cardiovascular death independently of SCORE, the combination of all markers and SCORE only resulted in insignificant reclassification of subjects and mainly moved subjects without events into lower-risk categories. However, in a risk model where subjects with 1%≤SCORE<5% and presence of subclinical organ damage recommended treatment, the sensitivity of SCORE was increased from 65% to 89% (p<0.05) whilst specificity dropped from 81% to 57% (p<0.05).
Figure 1. Event rates per 1000 person-years of a composite cardiovascular endpoint consisting of cardiovascular and non-fatal myocardial infarction and stroke in groups divided according to recommendations on antihypertensive treatment from the European Society of Hypertension (ESH) or the European Society of Cardiology (ESC) and SCORE. Data from 1344 apparently healthy subjects with higher than optimal blood pressure in a Danish population study. The ESH risk chart recommends antihypertensive treatment in healthy individuals if blood pressure ≥140/90 mmHg or ≥130/85 mmHg plus presence of at least three additional risk factors, metabolic syndrome or subclinical organ damage. SCORE recommends antihypertensive treatment if SCORE≥5%, blood pressure ≥160/100 mmHg or blood pressure ≥140/90 mmHg if 1%≤SCORE<5%. The number of events and subjects in subgroups are depicted on top of bars. Reproduced with permission from Sehestedt et al. (Citation42)
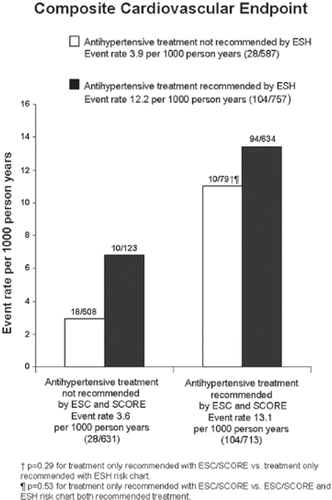
Figure 2. The cumulative probability (%) and hazard ratios of cardiovascular death in subgroups, according to SCORE and presence of subclinical organ damage in a Danish population sample of 1968 apparently healthy subjects. Dotted lines denote the 10 year 5% cumulative probability of cardiovascular death. Subjects with SCORE<5% and no subclinical organ damage were the reference group. SOD, subclinical organ damage. The different types were: left ventricular hypertrophy (LVH), atherosclerotic plaques, pulse wave velocity (PWV) >12 m/s and urine albumin/creatinine ratio (UACR) ≥90th percentile. Reproduced with permission from Sehestedt et al. (Citation43)
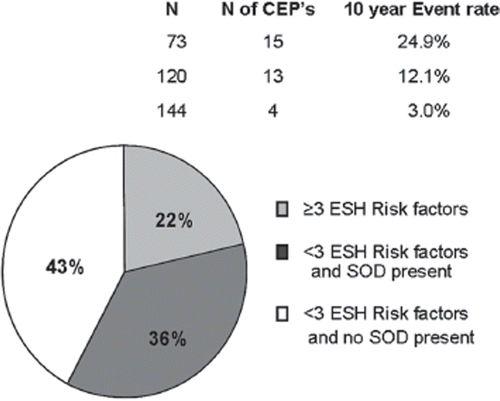
Which and how many markers to measure? The total number of markers of subclinical organ damage is overwhelming. However, although the different markers measure different intermediate endpoints, the risk information will to some degree overlap between markers because they reflect shared pathways in the pathogenesis of cardiovascular disease. Few studies have performed head-to-head comparisons of different markers of subclinical organ damage in the general population. In the Multi-Ethnic Study of Atherosclerosis, prediction of a composite cardiovascular endpoint with CAC was compared with IMT adjusted for traditional risk factors in healthy subjects from the general population. CAC was found to have a slightly better area under the ROC than IMT: 0.81 (95% CI 0.78–0.83) vs 0.78 (95% CI 0.75–0.81) (Citation44). We examined 337 healthy subjects with high normal blood pressure in the previously described cohort study. Healthy subjects in this blood pressure category are only considered at high added risk in the ESH guidelines if they have three or more traditional risk factors, metabolic syndrome or subclinical organ damage. As illustrated in , presence of subclinical organ damage did indeed identify a high-risk group in the group with fewer than three traditional risk factors. Using PWV, LVMI, carotid plaques as well as UACR increased the sensitivity of the ESH risk chart from 47% to 88% (p<0.001) and the proportion of individuals in whom antihypertensive drug treatment was recommended from 22% to 57% (p<0.001) compared with the ESH chart with no measurements of subclinical organ damage (Citation45). However, using two of UACR, PWV or plaques did not produce significantly worse results as measuring all four markers, indicating that a smaller selection of markers would suffice in order to increase risk prediction.
Figure 3. Three hundred and thirty-seven apparently healthy subjects with high normal blood pressure in a Danish population sample divided into groups by number of risk factors in European Society of Hypertension (ESH) risk classification chart and whether or not subclinical organ damage (SOD) was present and the corresponding 10-year event rates of the composite cardiovascular endpoint (CEP) consisting of cardiovascular death and non-fatal myocardial infarction and stroke. Percentage of total number of each subgroup is denoted inside circle. Reproduced with permission from Sehestedt et al. (Citation45).
True assessment of the impact of measuring subclinical organ damage as well as the choice between markers will have to take into account considerations about cost and availability. There are great variations between markers, from the cheap and quick ECG or urine sample to costly and time-consuming markers such as echocardiography or CAC score. Furthermore, inclusion of new markers will often lead to lower specificity and a higher number of false positive test results (Citation45), which could lead to unnecessary anxiety as well as increased cost because of increased medication in patients. However, with the reduced cost of effective and safe medication for primary prevention such as statins, this could be perceived as acceptable. Indeed, from a cost benefit point of view some may argue that if the cost of statins continues to drop, an unconditional use, i.e. without extensive testing, might be considered (Citation46). Finally, the risk of false reassurance because of a false-negative test result as well as the risk of examination as in the case of radiation exposure with computer tomography should be taken into consideration.
Routine use of markers of subclinical organ damage. There is to date a lack of comprehensive knowledge of the costs associated with measurements of subclinical organ damage. Consequently, it is still heavily disputed whether subclinical organ damage should be measured as routine. Some argue for screening all middle-aged asymptomatic subjects (Citation47), while most argue that screening should not be encouraged (Citation48–50), based on the insufficient data regarding the direct and indirect costs of screening, lack on consensus on thresholds for the individual markers and the lack of studies showing considerably incremental risk prediction beyond that of traditional risk factors. The ESH recommends considering routine measurements of urine protein excretion, estimated GFR with the MDRD formula and ECG in hypertensive individuals because of their simplicity, wide availability and limited cost (Citation6). Our studies did not demonstrate significant clinical improvements in risk stratification in healthy subjects, comparing the ESH risk chart with its elaborate measurements of subclinical organ damage with a simple risk equation based on easily available risk factors such as SCORE, and consequently did not support the routine use in the general population (Citation42). However, as we were able to demonstrate an improved risk prediction when information on subclinical organ damage was added to SCORE in subjects with 1%≤SCORE<5% (Citation43) as well as subjects with high normal blood pressure (Citation45), there might be a role for screening in these intermediate risk groups.
Beyond risk prediction. Measuring subclinical organ damage could have benefits beyond evaluating initial risk. In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study with more than 9000 patients with hypertension and LVH, UACR was measured at baseline and annually (Citation51). When UACR decreased during treatment, cardiovascular risk was also reduced and this change was not explained by in-treatment level of blood pressure. Consequently, UACR could be used to monitor the effect of treatment identifying non-responders that might have benefit of intensified treatment, even though the initial blood pressure goal had been achieved. Similar results were seen in the LIFE study regarding in-treatment changes of LVH assessed by echocardiography (Citation52) as well as ECG (Citation53).
In-treatment changes in IMT in the European Lacidipine Study on Atherosclerosis (ELSA), a large, randomized, intervention trial in 2334 hypertensive individuals did not predict cardiovascular outcomes, whereas baseline IMT did (Citation22). However, the treatment induced IMT changes were much smaller than the differences between individual baseline values, which may explain this negative result.
It has also been hypothesized that the measurements of subclinical organ damage can serve as a motivational tool. Patients who are confronted with for instance an image of a carotid artery with atherosclerotic plaques would according to this hypothesis be more likely to change lifestyle behavior or increase their medication compliance. Only one randomized controlled study has tested this hypothesis in a group of 450 healthy young subjects who were examined with electron beam tomography and randomized to receive scanning results (Citation54). In this low-risk group, there was no difference in the Framingham risk score after 1 year follow-up between those who were provided a test result and those who were not. However, there was an insignificant trend towards risk reduction in the subgroup that had an abnormal calcium scan.
Several studies without control groups have investigated behavioral changes in asymptomatic subjects referred to electron beam tomography assessment by their physician (Citation55,Citation56). The participants were informed of their scanning results and after follow-up the group with the highest CAC score demonstrated a 2–3 times greater usage of aspirin and lipid lowering medications as well as an improvement in exercise and diet compared with the group with the lowest score.
Conclusion
Measuring subclinical organ damage is a theoretically attractive way to improve cardiovascular risk prediction, to individualize risk assessment and to monitor whether treatment of the traditional risk factors have impact on the cardiovascular system. However, to assess the clinical impact we need randomized controlled studies in which patients are treated with or without use of markers of organ damage investigating increments in discrimination and calibration, number of clinical significant reclassifications and economic cost versus health benefit. These studies should focus on subjects with intermediate risk, such as subjects with high normal blood pressure or 1%≤SCORE<5%, because reclassification in this group will have the greatest impact on risk stratification and treatment recommendations.
Acknowledgements
The work of Thomas Sehestedt was supported by a fellowship from the European Society of Hypertension.
Declaration of interest: None declared.
References
- Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: Time to end the “only-50%” myth. Arch Intern Med. 2001;161:2657–2660.
- Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, . Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: Findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282: 2012–2018.
- Law MR, Wald NJ, Morris JK. The performance of blood pressure and other cardiovascular risk factors as screening tests for ischaemic heart disease and stroke. J Med Screen. 2004;11:3–7.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421.
- Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, . Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertens. 2009 Oct 15.
- Bombelli M, Facchetti R, Carugo S, Madotto F, Arenare F, Quarti-Trevano F, . Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens. 2009;27:2458–2464.
- Woythaler JN, Singer SL, Kwan OL, Meltzer RS, Reubner B, Bommer W, . Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: Comparison with postmortem mass measurements. J Am Coll Cardiol. 1983;2:305–11.
- Myerson SG, Montgomery HE, World MJ, Pennell DJ. Left ventricular mass: Reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension. 2002;40:673–678.
- Verdecchia P, Angeli F, Cavallini C, Mazzotta G, Repaci S, Pede S, . The voltage of R wave in lead aVL improves risk stratification in hypertensive patients without ECG left ventricular hypertrophy. J Hypertens. 2009;27:1697–1704.
- Li Z, Dahlof B, Okin PM, Kjeldsen SE, Wachtell K, Ibsen H, . Left bundle branch block and cardiovascular morbidity and mortality in hypertensive patients with left ventricular hypertrophy: The Losartan Intervention For Endpoint Reduction in Hypertension study. J Hypertens. 2008;26: 1244–1249.
- Schillaci G, Pirro M, Ronti T, Gemelli F, Pucci G, Innocente S, . Prognostic impact of prolonged ventricular repolarization in hypertension. Arch Intern Med. 2006;166:909–13.
- Okin PM, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Nieminen MS, . Prognostic value of changes in the electrocardiographic strain pattern during antihypertensive treatment: The Losartan Intervention for End-Point Reduction in Hypertension Study (LIFE). Circulation. 2009;119: 1883–1891.
- Havranek EP, Emsermann CD, Froshaug DN, Masoudi FA, Krantz MJ, Hanratty R, . Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342–350.
- Zanchetti A, Cuspidi C, Comarella L, Rosei EA, Ambrosioni E, Chiariello M, . Left ventricular diastolic dysfunction in elderly hypertensives: Results of the APROS-diadys study. J Hypertens. 2007;25:2158–2167.
- Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, . Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens. 2005;23:183–91.
- Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351.
- Okin PM, Roman MJ, Lee ET, Galloway JM, Howard BV, Devereux RB. Combined echocardiographic left ventricular hypertrophy and electrocardiographic ST depression improve prediction of mortality in American Indians: The Strong Heart Study. Hypertension. 2004;43:769–774.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467.
- Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Lochen ML, . Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: A 6-year follow-up study of 6226 persons: The Tromso Study. Stroke. 2007;38:2873–2880.
- Touboul PJ, Labreuche J, Vicaut E, Amarenco P. Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005;36: 1741–1745.
- Zanchetti A, Hennig M, Hollweck R, Bond G, Tang R, Cuspidi C, . Baseline values but not treatment-induced changes in carotid intima-media thickness predict incident cardiovascular events in treated hypertensive patients: Findings in the European Lacidipine Study on Atherosclerosis (ELSA). Circulation. 2009;120:1084–1090.
- Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27.
- Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: A tool for rapid evaluation of new therapies. Stroke. 2005;36:1904–1909.
- Laurent S, Cockcroft J, Van BL, Boutouyrie P, Giannattasio C, Hayoz D, . Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, . Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208.
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, . Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345.
- Part 4. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis. 2002;39(2, Supplement 1): S46–S75.
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, . Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315.
- Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, . Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002;62:1402–1407.
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, . Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100.
- Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44:84–93.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Ruilope LM, Zanchetti A, Julius S, McInnes GT, Segura J, Stolt P, . Prediction of cardiovascular outcome by estimated glomerular filtration rate and estimated creatinine clearance in the high-risk hypertension population of the VALUE trial. J Hypertens. 2007;25:1473–1479.
- Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant. 2005;20:1791–1798.
- Cirillo M, Lanti MP, Menotti A, Laurenzi M, Mancini M, Zanchetti A, . Definition of kidney dysfunction as a cardiovascular risk factor: Use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med. 2008;168:617–624.
- Olsen MH, Wachtell K, Ibsen H, Lindholm LH, Dahlof B, Devereux RB, . Reductions in albuminuria and in electrocardiographic left ventricular hypertrophy independently improve prognosis in hypertension: The LIFE study. J Hypertens. 2006;24:775–781.
- Jensen JS, Feldt-Rasmussen B, Strandgaard S, Schroll M, Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35: 898–903.
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935.
- Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172.
- Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De BG, . Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur Heart J. 2003;24:987–1003.
- Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, . Risk stratification with the risk chart from the European Society of Hypertension compared with SCORE in the general population. J Hypertens. 2009;27:2351–2357.
- Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Petersen C, . Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2009;Dec 23.
- Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, . Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168: 1333–1339.
- Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, . Which markers of subclinical organ damage to measure in individuals with high normal blood pressure? J Hypertens. 2009;27:1165–1171.
- Diamond GA, Kaul S. The things to come of SHAPE: Cost and effectiveness of cardiovascular prevention. Am J Cardiol. 2007;99:1013–1015.
- Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, . From vulnerable plaque to vulnerable patient–Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H.
- Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G. The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: Systematic review. Health Technol Assess. 2006;10:iii–x, 1.
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, . ACCF/AHA. 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the. 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation. 2007;115:402–426.
- Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, . Emerging risk factors for coronary heart disease: A summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507.
- Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, . Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202.
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, . Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349.
- O'Malley PG, Feuerstein IM, Taylor AJ. Impact of electron beam tomography, with or without case management, on motivation, behavioral change, and cardiovascular risk profile: A randomized controlled trial. JAMA. 2003;289: 2215–2223.
- Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis. 2006;185:394–399.
- Orakzai RH, Nasir K, Orakzai SH, Kalia N, Gopal A, Musunuru K, . Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. Am J Cardiol. 2008; 101:999–1002.