Abstract
Background. Cardiovascular diseases are accompanied by the presence of active oxygen species and organic free radical generation. The aim of this study was to examine the possibility of using malondialdehyde (MDA)-modified low-density lipoprotein (LDL) analyses as a diagnostic and prognostic biomarker. Design and methods. A cross-sectional epidemiological study of a random sample of the male population of Tallinn aged 20–64 was carried out in 2007–2008. A total of 413 subjects were included in the study. The screening procedure included standard epidemiological methods. Oxidative stress was assessed by measuring the kinetics of glutathione oxidation and the level of oxidized of MDA-modified LDL. Results. A strong positive correlation between levels of MDA-modified LDL- and total cholesterol was indicated, as well as LDL-cholesterol in blood of patients with postinfarct cardiosclerosis (r=0.82 and r=0.83, respectively, p<0.05). Hypercholesterolemia and hyperglyceridemia were accompanied by significant increase in oxidized LDL plasma level. Conclusion. MDA-modified LDL estimation has a diagnostic accuracy and may be used as an independent biochemical marker for atherosclerosis.
Introduction
The development of cardiovascular and other pathology is accompanied by a disturbance in the regulation of free radical peroxidation processes leading to oxidative stress in the cells and tissues (Citation1,Citation2). Cardiovascular diseases and many other disorders, such as diabetes mellitus, neurodegenerative diseases etc., are accompanied by a generation of active oxygen species and organic free radicals (Citation2–4).
Atherogenic low-density lipoproteins (LDL) are blood plasma lipoproteins that are most sensitive to induction of free radical oxidation (Citation3,Citation4). It was shown that peroxidation of the outer layer phospholipids of LDL particles is followed by conformational changes in the basic LDL protein apoprotein B-100 (apoB-100) and its displacement from the hydrophobic zone of the particle into the aqueous phase (Citation3). This can increase non-receptor uptake of LDL by the cells of blood vessel walls (Citation3). Carbonyl-containing compounds, such as malondialdehyde (MDA) and α,β-unsaturated aldehydes (4-hydroxynonenal and 4,5-dihydroxydecenal), generated during oxidative destruction of lipid hydroperoxides, can react with amino groups of proteins and produce strong intermolecular cross-links of the Schiff base type (Citation4). Similarly, aldehydes generated during homolysis of lipid hydroperoxides into oxidized LDL (oxLDL) form adducts with ϵ-amino groups of lysine residues of apoB-100 molecules that leads to changes in LDL conformation (Citation4). Modified LDL are recognized by “scavenger receptors” of blood vessel wall macrophages and actively captured by these cells. MDA-modified LDL can also be recognized by other receptors of macrophages, which do not compete with scavenger receptors and may be named “oxLDL receptors” (Citation4). On intensive absorption of oxLDL, macrophages transform to lipid-overloaded “foam cells” and secrete a factor that stimulates their colony formation. It results in clustering of cells with lipid inclusions and generation of primary pre-atherogenic damage to the blood vessel wall as fatty streaks (Citation3,Citation4). Thus, oxidative stress accompanied by intensification of free radical oxidation of lipids in biomembranes and LDL of blood plasma will promote development and manifestation of atherosclerosis (Citation2). Our investigations showed that the presence of risk factors such as hypercholesterolemia or type II diabetes mellitus stimulated accumulation of oxLDL as well as diabetic hyperglycemia in patients with atherosclerosis and significantly increased the oxLDL level in blood plasma of patients (Citation5–7).
We showed that estimation of oxLDL level in blood plasma of patients had a higher diagnostic accuracy than estimation of other biomarkers such as total cholesterol (TC), LDL-cholesterol (LDL-C) levels, lipoprotein-associated phospholipase A2 as well as high-density lipoprotein (HDL)-cholesterol (HDL-C) level (Citation7–11). Hence, the aim of the present communication was to investigate the possibility of using MDA-modified LDL estimation for independent assessment of patients with cardiovascular diseases in an epidemiological sample of population in Tallinn (Estonia), as well as investigate the possibility of using MDA-modified LDL analysis as an additional biomarker for cardiovascular risk.
Material and methods
Sampling and recruitment
The study was a cross-sectional epidemiological survey of an independent random sample of the male population of Tallinn (permanent residents of the city) aged 20–64, and was carried out in 2007–2008. The sample for the survey was drawn from the Estonian Population Register, which was established on the basis of the electoral list by a computer program. The calculation of the sample size was based on the protocol of the WHO/Countrywide Integrated Noncommunicable Disease Intervention (CINDI) Programme (Citation12). The participants were invited by mail, with up to three follow-up letters for non-respondents. A total number of 413 subjects were included in the study.
Clinical measurements
The screening procedure included the Rose questionnaire for determination of angina on effort, blood pressure, height and weight measurements with calculation of the BMI (weight/height2, kg/m2), determination of TC, triglycerides (TG), HDL-C, LDL-C, apolipoproteins AI (ApoLpA1) and B (ApoLpB), lipoprotein (a) [Lp(a)], glucose and homocysteine in blood plasma. Blood pressure was measured after a 5 min rest on the right arm with a mercury sphygmomanometer and recorded to the nearest 2 mm. Diastolic blood pressure (DBP) was recorded by the Korotkoff fifth phase. The mean of the two blood pressure readings was used in this study. Weight was measured in the participant without shoes and heavy outer garments, and recorded to the nearest 100 g. Height was recorded to the nearest 0.5 cm. The questionnaire also included questions and criteria on smoking habits, alcohol consumption, physical activity and other risk factors. The participants were also asked about previous history of hypertension and diabetes mellitus, awareness on elevated blood pressure and treatment. A subject was considered hypertensive if SBP ≥ 140 and/or DBP ≥ 90 mmHg (or on antihypertensive medication). A subject was considered a smoker if he had smoked at least 1 cigarette every day for the last 12 months. Overweight was defined as BMI ≥ 27 kg/m2, hypercholesterolaemia as TC ≥ 5.0 mmol/l or LDL-C≥ C3.0 mmol/l, hypertriglyceridaemia as TG ≥ 1.7 mmol/l. Diabetes mellitus was defined if a patient had the diagnosis at the time of examination.
Blood samples
At every visit, a venous blood sample was taken for biochemical assessments. Venous blood samples were collected after a fast at least 12 h by standard methods from an antecubital vein (BD Vacutainer, Belliver Industrial Estate, Plymouth; Becton, Dickinson and Co., UK). Blood samples for determination of the special biomarkers were collected in vacutainers without any preservatives for studies of glucose, TC, HDL-C, LDL-C and TG. The blood samples were centrifuged and serum transported to the central laboratory within 2 h on ice. For plasma homocysteine (P-Hcy), apolipoproteins ApoLpA1, ApoLpB and Lp(a) determinations, whole blood was collected in Li-heparin collection tubes and was placed immediately on ice until separated by centrifugation within 30 min.
All determinations were performed in accordance with standard procedures on the day of collection within 4 h after sampling at the laboratory of Clinical Chemistry, North Estonian Regional Hospital. Blood samples for further determination of oxidative stress parameters were collected in EDTA vacutainers. Blood samples for determination glutathione peroxidase (GSH-Px) activity in erythrocytes were prepared to obtain lysis by mixing (1:9 v/v) the whole blood with 0.05 mM K,Na–phosphate buffer, pH 7.4. Lysates were divided into two aliquots and stored before measurements at –80°C (for no more than 1 month) (Citation13). The remaining EDTA blood was centrifuged at +4°C, plasma separated into two aliquots and stored at –80°C until immunoassay.
Assays
Glucose was determined by the enzymatic reference method with hexokinase, TC by a cholesterol oxidase technique, HDL-C and LDL-C by homogeneous enzymatic colorimetric assay, and TG by using a glycerol phosphate oxidase technique after enzymatic cleavage of fatty acids. All of these determinations were performed using reagents obtained from Roche (Roche Diagnostics, Mannheim), in a Roche Cobas 6000 analyzer. Plasma homocysteine was measured by Diazyme Enzymatic assay. Apolipoproteins ApoLpA1, ApoLpB and Lp(a) were analysed using immunoturbidimetric assays (Roche Diagnostics, Mannheim). The intensity of turbidity, proportional to the concentration of antibody–antigen complexes, was measured by the Roche Integra 400 analyzer.
For estimation of the content of 2-thiobarbituric acid-reactive substances (TBARS), 0.4 ml blood plasma was mixed with 0.8 ml 15% trichloracetic acid and 0.4 ml 0.67% 2-thiobarbituric acid and boiled for 45 min. After cooling up to room temperature, samples were centrifuged for precipitate formation and supernatants were measured at 535 nm with a Labsystems OY FP-901 chemical analyzer (Finland) (Citation14). The GSH-Px activity in lysates of red blood cells was determined at 25°C by measuring the kinetics of gluthatione oxidation at 340 nm (by NADPH oxidation in coupled gluthatione reductase reaction) with a Labsystems Oy FP-901 chemical analyzer (Finland) using tert-butylhydroperoxide as the substrate by method in our modification. The activity of GSH-Px necessary for oxidation of 1 μmol reduced gluthatione for 1 min was taken as an activity unit (Citation14). The level of oxidized LDL (MDA-modified LDL) was measured by oxidized LDL ELISA (Mercodia, Sweden) using an Absorbance Microplate Reader, BioTek EL 808 (Citation15). Plasma with EDTA was diluted with sample buffer in two steps to a final dilution of 1/6561. After that, 25 μl of each sample were pipetted into coated plate wells, then 100 μl of assay buffer was added. Coated plates were incubated on a plate shaker (700 rpm) for 2 h and then washed six times. After the washing, 100 μl of enzyme conjugate solution was added to each well, 200 μl of substrate TMB solution was added and the plate was incubated for 15 min without shaking; 50 μl of stop solution (0.5 M H2SO4) was added to complete the reaction. The optical density for each well was read at 450 nm. All test procedures were carried out at 25°C. The level of oxidized LDL in the sample was calculated relative to calibrators. The content of Hb in red blood cells was determined by hemoglobin cyanide method with Herbos Dijagnostica kit.
Gluthatione reductase, NADPH, reduced gluthatione, 2-thiobarbituric acid, EDTA, K2HPO4 and NaH2PO4 were purchased from Sigma Chemicals; tert-butylhydroperoxide was from Merck.
Results and discussion
Our data showed that there was a strong positive correlation (r=0.90; p<0.05) between TC and cholesterol of LDL levels in blood plasma of patients (). The results of MDA-modified LDL level in the groups of patients with hypercholesterolemia and hyperglyceridemia are presented in . The data show that hypercholesterolemia and hyperglyceridemia are accompanied by significant increases in oxidized LDL plasma levels. Thus, the data support the notion that free radical lipoperoxidation has an important role in the pathogenesis of atherosclerosis and diabetes mellitus (Citation4–6,Citation8). It should be stressed that the most advanced lipid peroxidation was found in groups of patients with disturbance of both lipid and carbohydrate metabolism, since hypercholesterolemia and diabetes are important risk factors of atherosclerosis development (Citation4–6,Citation8).
Figure 1. Correlation between total cholesterol (Chol) and cholesterol of low-density lipoprotein (LDL-Chol) levels in blood plasma of patients in investigated population (r=0.90; p<0.05).
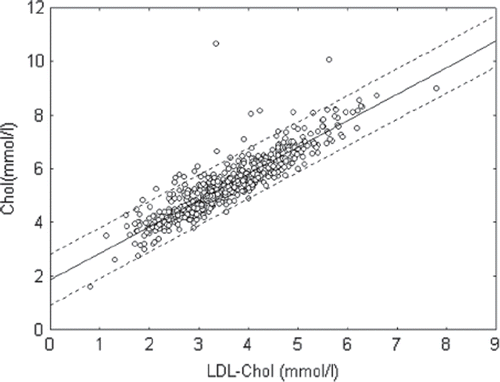
Table I. Level of oxidized low-density lipoprotein (LDL; MDA-modified LDL) in patients with and without hyperchole-sterolemia and hyperglyceridemia.
Currently, MDA-modified LDL is considered to be an important biomarker for cardiovascular disease, especially atherosclerosis (Citation7,Citation9–11,Citation16). Our data showed a positive correlation (r=0.52; p<0.05) between the presence of hypercholesterolemia and the level of oxLDL (MDA-modified LDL) in blood of patients. We also observed a weak correlation between the presence of hyperglyceridemia and the level of oxLDL in blood of patients (r=0.27; p<0.05). On the other hand, there was no correlation between the presence of hypercholesterolemia in plasma and GSH-Px activity in erythrocytes (r=–0.09; p>0.05), nor between the presence of hypercholesterolemia and the level of TBARS in plasma (r=0.01; p>0.05). Nevertheless, there was negative low correlation between the level of oxLDL and GSH-Px activity in erythrocytes (r=–0.19; p<0.05) but there was no correlation between the level of oxLDL and the level of TBARS in plasma of patients.
It is well known that the high level of TC and especially cholesterol of LDL in blood plasma of patients may be good biomarker for the presence of atherosclerosis (Citation3,Citation7,Citation10). We found that there was a strong positive correlation between the levels of TC or LDL-C and oxLDL (MDA-modified LDL) level (r=0.68 and 0.72 correspondently; p<0.05) in blood of patients from our population (, and ). In the group of patients with postinfarct cardiosclerosis, we observed a strong positive correlation between oxLDL and TC or cholesterol of LDL levels in blood plasma of patients (). In the group of patients with diabetes mellitus, these correlations were weaker (). This may be explained by the fact that patients with diabetes accumulate not only MDA but also glyoxal and other low molecular weight aldehydes, which compete with MDA for LDL modification (Citation4). Our preliminary data indicate that the antigen properties of apoB-100 in LDL for MDA were different from binding sites for glyoxal and other low molecular weight aldehydes, since monoclonal antibodies to MDA-modified LDL were not binding together with glyoxal-modified LDL (unpublished data). Furthermore, the strong positive correlation between oxLDL level and TC or cholesterol of LDL levels in blood of patients with hypertension was expected (). Correlations were also observed in groups of patients with risk factors such as overweight and smoking. In fact, there was a strong positive correlation between oxLDL and TC, as well as cholesterol of LDL levels, in blood plasma of overweight patients (), as well as between oxLDL and TC or cholesterol of LDL levels in smokers (). Taken together, our data support the notion that MDA-modified LDL estimation has an important diagnostic accuracy and may be used as an independent biochemical marker for atherosclerosis.
Figure 2. Correlation between oxidized low-density lipoprotein (oxLDL) and total cholesterol (Chol) level in blood plasma of patients in investigated population (r=0.68; p<0.05).
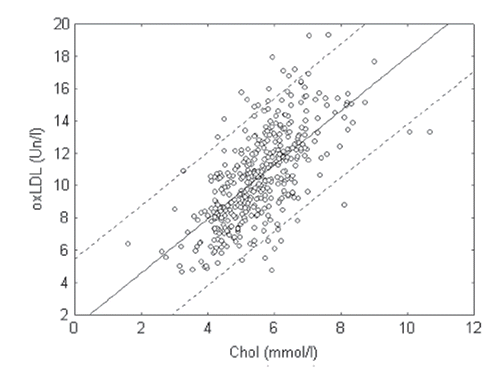
Figure 3. Correlation between oxidized low-density lipoprotein (oxLDL) (MDA-modified LDL) and cholesterol of LDL level (LDL-Chol) in blood plasma of patients in investigated population (r=0.72; p<0.05).
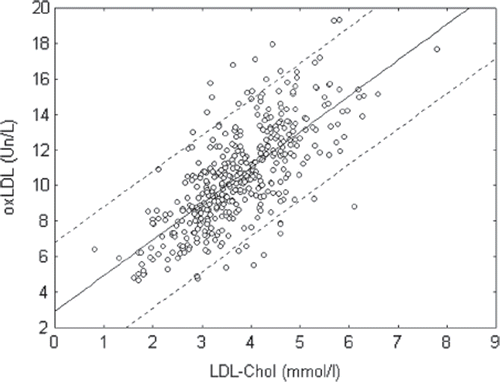
Table II. Correlation between oxidized low-density lipoprotein (oxLDL) and total cholesterol or cholesterol of LDL levels in blood serum in different groups of patients.
Acknowledgements
This study was supported by Estonian core grant SF0140027s07, Tallinn City Government grant, and by the European Union through the European Regional Development Fund.
Declaration of interest: None declared.
References
- Lankin V. The enzymatic systems in the regulation of free radical lipid peroxidation. Free radicals, nitric oxide, and inflammation: Molecular, biochemical, and clinical aspects. Amsterdam: IOS Press; 2003, NATO Science Series 344. 8–23.
- Lankin V, Tikhaze A. Free radical lipoperoxidation during atherosclerosis and antioxidative therapy of this disease. Free radicals, nitric oxide, and inflammation: Molecular, biochemical, and clinical aspects. Amsterdam: IOS Press; 2003, NATO Science Series 344. 218–231.
- Lankin VZ, Tikhaze AK, Kukharchuk VV, Konovalova GG, Pisarenko OI, Kaminnyi AI, . Antioxidants decrease the intensification of low density lipoprotein free radical peroxidation during therapy with statins. Mol Cell Biochem. 2003; 249 Suppl 1/2:129–140.
- Lankin VZ, Tikhaze AK, Kapel’ko VI, Shepel’kova GS, Shumaev KB, Panasenko OM, . Mechanisms of oxidative modification of low density lipoproteins under conditions of oxidative and carbonyl stress. Biochemistry. 2007;72 Suppl 10:1081–1090.
- Lankin VZ, Lisina MO, Arzamastseva NE, Konovalova GG, Nedosugova LV, Kaminnyi AI, . Oxidative stress in atherosclerosis and diabetes. Bull Exp Biol Med. 2005;140 Suppl 1:41–43.
- Nedosugova LV, Lankin VZ, Balabolkin MI, Konovalova GG, Lisina MO, Antonova KV, . Interrelation between compensation of carbohydrate metabolism and severity of manifestations of oxidative stress in type II diabetes mellitus. Bull Exp Biol Med. 2003;136 Suppl 2:132–134.
- Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Improved identification of patients with coronary artery disease by the use of new lipid and lipoprotein biomarkers. Am J Cardiol. 2006;97 Suppl 5:640–645.
- Girona J, Manzaranes JM, Marimon F, Cabre A, Heras M, Guardiola M, . Oxidized to non-oxidized lipoprotein ratios are associated with atherosclerosis and the metabolic syndrome in diabetic patients. Nutr Metab Cardiovasc Dis. 2007;18 Suppl 5:380–387.
- Imazu M, Ono K, Tadehara F, Kajiwara K, Yamamoto H, Sumii K, . Plasma levels of oxidized low density lipoprotein are associated with stable angina pectoris and modalities of acute coronary syndrome. Int Heart J. 2008;49 Suppl 5:515–524.
- Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, . Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis Circulation. 2008;118 Suppl 1:75–83.
- Rietzschei ER, Langlois M, De Buyzere ML, Segers P, De Bacquer D, Bekaert S, . Oxidized low-density lipoprotein cholesterol is associated with decreases in cardiac function independent of vascular alterations. Hypertension. 2008;52 Suppl 3:535–541.
- Protocol and Guidelines. Countrywide Integrated Noncommunicable Diseases Intervention (CINDI) Programme. Copenhagen: WHO Regional Office for Europe; 1996.
- Irodova NL, Lankin VZ, Konovalova GG, Kochetov AG, Chazova IE. Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med. 2002;133 Suppl 6:580–582.
- Arzamastseva NE, Lankin VZ, Konovalova GG, Tikhaze AK, Ageev FT, Lapina YV, . Oxidative stress in patients with chronic heart failure and type II diabetes mellitus. Bull Exp Biol Med. 2007;143 Suppl 2:207–209.
- Sigurdardottir V, Fagerberg B, Hulthe J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year old men (AIR Study). J Internal Med. 2002;252 Suppl 2:440–447.
- Verhoye E, Langlois MR. Circulating oxidized low-density lipoprotein: A biomarker of atherosclerosis and cardiovascular risk. Clin Chem Lab Med. 2009;47 Suppl 2:128–137.