Abstract
Aims. The aim of this investigation was to study the effects of hormone replacement therapy (HRT) on left ventricular mass (LVM) and serum-angiotensin-converting enzyme (ACE) activity (S-ACE) in well controlled hypertensive postmenopausal women. Methods. In this prospective, randomized, crossover, double-blind trial we studied 20 well controlled hypertensive postmenopausal women who received 6 months of HRT and 6 months of placebo on top of antihypertensive treatment. Two-dimensional M-mode, office blood pressure, 24-h ambulatory blood pressure (ABPM), S-estradiol and S-ACE activity were investigated at baseline, after 6 and 12 months. Results. LVM was significantly influenced by HRT (analysis of variance, ANOVA, p<0.01). However, the order in randomization of HRT and placebo had an impact on the analysis of LVM reduction (baseline – HRT – placebo: ns; baseline – placebo – HRT: p<0.01 ANOVA). Only the women lacking blockade of the renin–angiotensin–aldosterone system (RAAS) as antihypertensive treatment (n=10) experienced a reduction in LVM and a tendency of decreased S-ACE activity in response to HRT compared with baseline (p< 0.05 and p= 0.06 respectively). Conclusions. Six months of HRT resulted in significant reduction of LVM without any change in ABPM. HRT may reduce LVM through interaction with the RAAS, since hypertensive women without RAAS blockade exhibited an effect of HRT on LVM and S-ACE activity, which was not seen in women on RAAS blockade.
Key Words::
Introduction
A substantial proportion of global disease burden is attributable to high blood pressure (Citation1). Prior to menopause, the prevalence of hypertension is higher in men than in women, but later in life the rise in systolic blood pressure (SBP) is steeper in women than in men, and overall more women than men are treated for hypertension (Citation2). This could imply an effect of reproductive hormones on blood pressure regulation. In some studies, menopause has been found to contribute to an increase in the prevalence of hypertension, but the change in blood pressure is not as prominent as the related changes in lipids, and it is also difficult to separate the effects of menopause from the age-related changes in blood pressure (Citation3). It is well known that hypertension is associated with an increase in left ventricular mass (LVM) both through hemodynamic and non-hemodynamic factors (Citation4,Citation5). Left ventricular hypertrophy (LVH) is associated with an important increase in cardiovascular risk for both men and women (Citation6) and reversal of LVH appears to improve cardiovascular prognosis but there might exist gender differences regarding this aspect (Citation7,Citation8).
Several investigators have studied the effects of different estrogen and progesterone compounds on blood pressure and cardiac structure. The PEPI trial could not prove any significant effects of oral hormonal replacement therapy (HRT) on blood pressure in normotensive women during chronic treatment (Citation9). However, we have previously reported a blood pressure reducing effect after 24-h estrogen replacement therapy (ERT), transdermally administrated, to hypertensive postmenopausal women (Citation10). Schillaci and coworkers addressed the question of estrogen-related effects on left ventricular structure as they found an increase in left ventricular wall thickness and midwall shortening related to menopausal status, which was aggravated among hypertensive postmenopausal women (Citation11). Improvement in left ventricular diastolic function in response to estrogen has also been found in healthy postmenopausal women (Citation12). Direct effects of estrogen on the myocytes and fibroblasts, possibly through estrogen receptor-related mechanisms, indicate effects of steroid hormones on the cardiac structure (Citation13,Citation14).
The renin–angiotensin–aldosterone system (RAAS) is an important system involved in both pressure control and the development and maintenance of LVH (Citation15). Hayward and coworkers put forward the hypothesis of a non-cardiac hormone modulating effect of left-ventricular mass, and already 12 years ago Proudler found that HRT could induce a decrease in serum-angiotensin-converting enzyme (S-ACE) activity (Citation16,Citation17). These findings are well in line with the positive relation between estrogen compounds and changes in angiotensin and S-ACE activity found during recent years (Citation18).
The present study was initiated to investigate if 6-month treatment with oral HRT, of the type used in observational studies in which cardioprotection has been suggested, could reduce LVM in well controlled hypertensive postmenopausal women when given on top of current antihypertensive therapy. Furthermore, we aimed to evaluate if the effect of HRT on LVM could involve RAAS-dependent mechanisms, e.g. if steroid hormones act as ACE inhibitors.
Material and Methods
Subjects
Twenty well controlled hypertensive postmenopausal women (mean 56 years, range 51–65 years) with mean ambulatory 24-h blood pressure 128/78 mmHg were recruited through advertisement in the population of Gothenburg, Sweden. All participants were non-smokers without diabetes or hyperlipidemia and had no history or heredity for cancer or thromboembolic disease. All of the participants had been on the same antihypertensive therapy with well-controlled blood pressure (<140/90 mmHg) for at least 1 year. We intentionally included both patients with blockade of the RAAS and patients without these types of antihypertensive treatment (50% from each group). At baseline, subjects were treated with one to two antihypertensive drugs and half of the group received RAAS-blocking therapy (ACE inhibitors or angiotensin receptor blockade). Furthermore, 36% of the patients were on beta-blocking treatment, 12% received calcium-channel blocking therapy and 12% were treated with diuretics. Mean body mass index (BMI) was 27.6 kg/m2 (range 17–32 kg/m2). All subjects were postmenopausal for at least 2 years prior to the study, and had not been on hormonal therapy for the last 6 months prior to the start of the study. None of the participants was a shift worker. Before inclusion, all women underwent a gynecological (pelvic) examination and mammography. Informed consent was obtained from each subject and the study was approved by the ethics committee of Göteborg University and performed in accordance with the Helsinki Declaration of Health.
Study design
In this randomized, double-blind, crossover, placebo-controlled study, each subject received 6 months of oral HRT (0.625 mg conjugated estrogen+0.5 mg medroxyprogesteronacetat; Premelle®) and 6 months of placebo. All patients were controlled at baseline, after 6 and 12 months.
The subject entered the laboratory in the morning after 12 h fasting. After verbal information, a venous cannula was inserted into an antecubital vein (venflon; Viggo, Helsingborg, Sweden) and the subject was left alone in supine position in a quiet room for 30 min. Office blood pressure was recorded in accordance with recommendations from the Swedish Association of Hypertension with a sphygmomanometer (Tonometer model minimus; Speidler and Keller, Junngingen, Germany), and the mean of three measurements was used for calculations. Blood samples were obtained without stasis from the indwelling venous cannula. Samples for analyses of S-ACE activity were immediately placed on ice, centrifuged at −4°C at 2000g for 5 min and placed in a freezer at −70°C The investigation was finished with a 24-h ambulatory blood pressure and heart rate monitoring (Spacelab 90202) where registrations were performed three times an hour between 06:00 and 24:00 h, and twice an hour between 00:00 and 06:00 h.
On day two, the ambulatory advice was removed and followed by an echocardiography. Two-dimensional guided M-mode was performed using Siemens Sequoia® 256 system (Siemens, Munich, Germany).
All examinations and all measurements were made by one, well experienced echo technician, blinded to treatment. Left ventricular dimensions were measured in end diastole, and LVM and LVM index (LVMI) were calculated according to the American Society of Echocardiography recommendations (Citation19,Citation20). The relative wall thickness (RWT) was calculated according to the formula: (septal thickness+posterior wall thickness)/left ventricular diameter (Citation21). The left ventricular volumes and ejection fraction were calculated according to the Teicholz formula (Citation22).
Patients were seen at the outpatient clinic every third month, and we aimed at keeping the office blood pressure constant during the study to make it possible to evaluate the non-blood pressure-related effects of HRT on LVM.
Analysis
S-ACE activity was analyzed by radioenzymatic assay, reference values 12–68 units/l (ACE direct, Bühlman Laboratories, AG, Schönenbuch, Switzerland).
Statistics
Subjects were used as their own controls.
Our prespecified aim was to compare the two equal subgroups of patients with and without antihypertensive treatment including drugs blocking the RAAS.
The power calculation regarding study size was based on the assumption of an ACE inhibiting effect of estrogen in the range of a regular ACE inhibitor (Citation23). We aimed at detecting a difference in LVM of 15% from baseline (with a standard deviation of the change by 36.7). This resulted in a study population of 22 individuals treated for 6 months with placebo and active treatment (80% power to detect a difference between active treatment and placebo).
Echocardiographic data were analyzed by two-way analysis of variance (ANOVA) for repeated measurements taking into account subject, treatment and time, and with Students t-test.
Ambulatory monitoring data were calculated as 1-h means of heart rate, SBP and diastolic blood pressure (DBP) and analyzed by two-way analysis of variance (ANOVA) for repeated measurements taking into account subject, treatment and time. The day period was defined as 06:00–23:00 h and the night period as 23:00–06:00 h in accordance with the majority of the diary notes.
To analyse S-ACE activity and S-estradiol, comparisons between baseline, active treatment and placebo were performed by Students t-test. All tests were considered significant at p<0.05. Values are given as mean ± SEM.
Results
S-estradiol was increased from 0.045±0.014 nmol/l (baseline) to 0.120 ±0.063 nmol/l in response to HRT treatment (p< 0.01).
BMI was not significantly changed during the study period.
Office SBP, DBP and heart rate were not significantly changed in response to HRT. There were no significant effects of treatment on the 24-h ambulatory recordings, when analyzing the total 24-h means or when looking at mean pressure values for day or night (). However, there was a significant interaction between treatment and time on the 24-h ambulatory SBP curve during the morning hours (06.00 to 12.00 h) (p< 0.05) with a more flattened curve after HRT compared with baseline and placebo.
Table I. Diastolic (DBP) and systolic blood pressure (SBP) and heart rate (HR) (office and 24-h ambulatory), left ventricular mass (LVM), left ventricular mass index (LVMI) and serum angiotensin-converting enzyme activity (S-ACE) in 20 hypertensive postmenopausal women at baseline and after 6 months of hormone replacement therapy (HRT) or placebo.
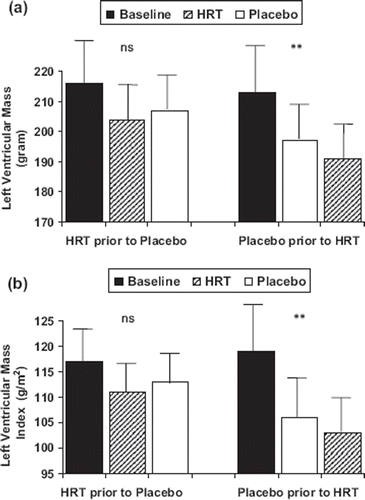
LVM and LVMI were significantly influenced by treatment (ANOVA p<0.01). Both placebo and HRT induced a significant reduction in LVM and LVMI compared with baseline (t-test p <0.05 for all) (). However, the order in randomization between HRT and placebo had an impact on the analysis of LVM () and LVMI () reduction (baseline – HRT – placebo: ns; baseline – placebo – HRT: p < 0.01 ANOVA) ( and ). However, the analysis of order in randomization did not visualize any significant effects of therapy on office blood pressure or ambulatory blood pressure monitoring.
Figure 1. (a) Left ventricular mass (LVM) in 20 hypertensive postmenopausal women at baseline and after 6 months treatment with hormone replacement therapy (HRT) or placebo. Values are given as mean±SEM. HRT prior to placebo (n = 10) represents patients where HRT was given prior to placebo. Placebo prior to HRT (n=10) represents patients where treatment with placebo was introduced in advance of HRT. (b) Left ventricular mass index (LVMI) in 20 hypertensive postmenopausal women at baseline and after 6 months treatment with hormone replacement therapy (HRT) or placebo. Values are given as mean±SEM. HRT prior to placebo (n = 10) represents patients where HRT was given prior to placebo. Placebo prior to HRT (n=10) represents patients where treatment with placebo was introduced in advance of HRT. ANOVA **p< 0.01 for influence of treatment, ns, not significant.
No significant effects of HRT compared with baseline or placebo were found with respect to other echocardiographic measurements performed in the current study.
HRT did not produce any significant affect on the levels of S-ACE activity, when analyzing the total study group ().
All patients were on antihypertensive therapy at baseline, and half the study population did not receive RAAS blockade as a part of their antihypertensive treatment (non-RAAS, n=10). In these women, LVM and LVMI were reduced in response to HRT (p<0.05) without any change after placebo treatment (). Conversely no significant effects of HRT was noted in patients on RAAS blockade (plus RAAS, n=10) ().
Table II. Office diastolic (DBP) and systolic blood pressure (SBP), left ventricular mass (LVM), left ventricular mass index (LVMI), serum angiotensin-converting enzyme activity activity (S-ACE) in 10 hypertensive postmenopausal women on renin–angiotensin–aldosterone system (RAAS) blockade and 10 hypertensive postmenopausal women without RAAS blockade at baseline and after 6 months of hormone replacement therapy (HRT) or placebo.
Furthermore, in non-RAAS subjects, we found a trend towards lower level of S-ACE activity after HRT treatment (p=0.06), which was not seen after placebo. On the contrast, HRT did not generate any effect on S-ACE activity in patients on RAAS blocking therapy (). The two subgroups (non-RAAS vs plus RAAS) did not differ with respect to BMI or blood pressure (). However, the non-RAAS subgroup showed a non-significant (13/10 mmHg) reduction of blood pressure after HRT, which was not the case in plus RAAS subjects.
Discussion
The present study demonstrates an effect of HRT on LVM and LVMI without any significant alterations in blood pressure. The order in randomization influenced the results and HRT only produced a decrease in LVM and LVMI when placebo was introduced prior to HRT. Interestingly, the effect of HRT on LVM and LVMI reduction was also influenced by the basal antihypertensive treatment with significant reduction only in women who did not receive RAAS blockade. Furthermore, in these women, HRT tended to reduce the level of S-ACE activity.
After 6 months intervention with placebo, a reduction of LVM and LVMI was present, and HRT reinforced this regression with a further decrease. This implies that HRT has an effect on cardiac mass beyond the alteration induced by placebo. Our results of an HRT-induced regression of LVM is strengthened by previous findings of a similar reduction in LVM found after 18 months treatment with transdermal ERT and placebo in a parallel-group design, although this decrease was accompanied by an immense reduction in blood pressure (Citation24,Citation25). The route of administration of estrogen has attracted much interest with respect to cardiovascular effects and as previously mentioned, our results are well in line with investigations using a transdermal formula of ERT (Citation24).
A decrease of LVM without any significant reduction in SBP or DBP may suggest that we should look for a non-blood pressure-related mechanism to explain our findings. In a recent review on the effects of hormone therapy on blood pressure and the RAAS in postmenopausal women, Prime and coworkers indentified several studies where different components of the system including bradykinin are affected by HRT without any significant effect on blood pressure levels (Citation26). Miya and coworkers found a reduction of LVM in response to oral treatment with HRT in an open trial without placebo control, where hypertensive women with LVH were investigated, and put forward several growth-promoting factors as explanatory cause (Citation27).
The hypothesis of an estrogen effect on LVM mediated by blockade of the RAAS emerges from work by Proudler and co-workers presented in the Lancet in 1995 (Citation17). More recent findings demonstrate that estrogen also antagonizes the growth-promoting effects of angiotensin II on vascular smooth muscle (Citation28). The neurohormonal response to HRT has been elucidated in several trials from Japan. These studies have shown a decrease in S-ACE activity and an increase in S-bradykinin in response to oral HRT, both in normotensive and hypertensive women. However, neither placebo control nor randomization or crossover design were used in these studies, and the correlation between changes in LVM and alteration in RAAS was not positive (Citation27,Citation29). Furthermore, the effect on RAAS in response to HRT in normotensive postmenopausal women could not be confirmed in the most recent publication (Citation30). The lack of conformity regarding study results may emerge from the diversity in study designs. The cardiovascular effects of steroid hormones are complex, and the importance of acute and chronic regimens of estrogen, different routes of administration and combinations with gestagens have attracted a large amount of interest (Citation31).
The hypothesis of a restraining influence of HRT on RAAS activity was addressed in the current study by a subgroup analysis. The women who did not receive antihypertensive treatment with an ACE inhibitor or an angiotensin receptor blocker showed a trend towards attenuated S-ACE activity after 6 months of HRT treatment but not after placebo. The same subgroup also demonstrated a reduction of LVM, and it is tempting to believe that this decrease is a result of a mild ACE-inhibiting effect of HRT and not necessarily mediated through a blood pressure reduction.
Our study has some limitations. Change in LVM develops over time and it is possible that the antihypertensive treatment interfered with the degree of LVM. A study design with a considerable washout period would probably have been appropriate, but it is unethical to perform an investigation in hypertensive patients for more than a year without any active treatment for blood pressure reduction. Furthermore, the study design was based on a basic scientific work regarding regress of LVM related to blockade of the RAAS (Citation32). It could be that estrogen acts as an ACE inhibitor, but the effect of combined oral HRT on LVM is probably less prominent than the effect of a regular ACE inhibitor. Therefore the duration of treatment might have been too short, or the study population, and especially the proportion of patients without RAAS blockade, might have been too small, which obviously leave us with a risk of a statistically Type II error. Finally, estrogen and progesterone have in some respects diverse effects on the cardiovascular system. This aspect could not be elucidated with this combined HRT.
In summary, we found that 6 months treatment with combined oral HRT on top of antihypertensive treatment in hypertensive postmenopausal women resulted in a small decrease in LVM without any significant effect on blood pressure. This is a novel finding as well as the finding of an effect of HRT on RAAS and LVM in a placebo/crossover design in hypertensive women without RAAS blocking treatment. The hypothesis of an ACE inhibiting effect related to oral HRT needs to be confirmed in a larger clinical trial.
Acknowledgements
We greatly acknowledge medical assistant Sten Eriksson and research nurse Margareta Ohlsson for excellent support during the performance of the study. We also want to acknowledge Organon AB for kindly providing us with active substances and placebo correlate. Part of this work was presented as an abstract at the American Society of Hypertension in San Francisco, 2005.
Conflict of interest: The study was performed on the initiative of the authors and we have no conflict of interest to declare.
References
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Comparative risk assessment group. Selected major risk factors Lancet. 2002;360:1347–1360.
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208.
- Staessen JA, Celis H, Fagard R. The epidemiology of the association between hypertension and menopause. J Hum Hypertens. 1998;12:587–592.
- Kannel WB. Left ventricular hypertrophy as a risk factor in arterial hypertension. Eur Heart J. 1992;13 Suppl D:82–88.
- Kahan T. The importance of left ventricular hypertropgy in human hypertension. J Hypertens. 1998;16 Supp 17:S23–29.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352.
- Verdecciha P, Schillaci G, Borgioni C, Ciuci A, Gattobigio R, Zampi I, . Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998; 97:48–54.
- Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation. 1994;90:928–36.
- Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995; 273:199–208.
- Manhem K, Ahlm H, Milsom I, Svensson A. Transdermal oestrogen reduces daytime blood pressure in hypertensive women. J Hum Hypertens. 1998;12:323–327.
- Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Porcellati C. Early cardiac changes after menopause. Hypertension. 1998; 32:764–769.
- Duygu H, Akman L, Ozerkan F, Akercan F, Zoghi M, Nalbantgil S, . Comparison of the effects of new and conventional hormone replacement therapies on left ventricular diastolic function in healthy postmenopausal women: A Doppler and ultrasonic backscatter study. Int J Cardiovasc Imaging. 2009;25:387–396.
- Grohé C, Kahlert S, Löbbert S, Stimpel M, Karas RH, Vetter H, . Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107–112.
- Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, . Estrogen receptor beta protects the murine heart against left ventricular hyperthrophy. Aterioscler Thromb Vasc Biol. 2006;26:1524–1530.
- Schmeider RE, Martus P, Klinbeil A. Reversal of left ventricular hypertrophy in hypertensive patients. A meta-analysis of randomized double-blind studies. JAMA. 1996;275: 1507–1513.
- Hayward CS, Webb C, Collins P. Effect of sex hormones on cardiac mass. Lancet. 2001;357:1354–1356.
- Proudler AJ, Ahmed AI, Crook D, Fogelman I, Rymer JM, Stevenson JC. Hormone replacement therapy and serum angiotensin-converting-enzyme activity in postmenopausal women. Lancet. 1995;346:89–90.
- Seely EW, Brosnihan KB, Jeunemaitres X, Okamura K, Williams GH, Hollenberg NK, . Effects of conjugated oestrogen and droloxifen on the renin–angiotensin system, blood pressure and renal blood flow in postmenopausal women. Clin Endocrinology. 2004;60:315–321.
- Sahn DJ. Recommendations regarding quantification in M-mode echocardiography. Results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083.
- Devereux RB. Echocardiographic assessment of left ventricular hypertrophy. C omparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, . Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. JACC. 1995;25: 871–878.
- Kronik G. Comparative value of eight M-mode ventricular echocardiographic formulas for determining left ventricular stroke volume. Circulation. 1979;60:1308–1316.
- Sanada M, Higashi Y, Nakagawa K, Sasaki S, Kodama I, Sakashita T, . Estrogen replacement therapy in postmenopausal women augments reactive hyperemia in the forearm by reducing angiotensin converting enzyme activity. Atherosclerosis.. 2001 Oct;158:391–397.
- Modena MG, Muia Jr N, Aveta P, Molinari R, Rossi R. Effects of transdermal 17β-estradiol on left ventricular anatomy and performance in hypertensive women. Hypertens. 1999;34:1041–1046.
- Modena MG, Molinari R, Muia Jr N, Castelli A, Pala F, Rossi R. Double-blind randomized placebo-controlled study of transdermal estrogen replacement therapy on hypertensive postmenopausal women. Am J Hypertens. 1999;12:1000–1008.
- Prime DD, Brosnihan KB, Herrington DM. Effects of hormone therapy on blood pressure and the renin–angiotensin system in postmenopausal women. Minerva Cardioangiol. 2007;55:477–485.
- Miya Y, Sumino H, Ichikawa S, Nakamura T, Kanda T, Kumakura H, . Effects of hormone replacement therapy on left ventricular hypertrophy and growth-promoting factors in hypertensive postmenopausal women. Hyptens Res. 2002;25:153–159.
- Takeda-Matsubara Y, Nakagami H, Iwai M, Cui TX, Shiuchi T, Akishita M, . Estrogen activates phosphatases and antagonizes growth-promoting effect of angiotensin II. Hypertension. 2002 Jan;39:41–5.
- Umeda M, Ichikawa S, Kanda T, Sumino H, Kobayashi I. Hormone replacement therapy increases plasma level of angiotensin II in postmenopausal hypertensive women. Am J Hypertens. 2001;14:206–211.
- Ichikawa A, Sumino H, Ogawa T, Ichikawa S, Nitta K. Effects of long-term transdermal hormone replacement therapy on the renin–angiotensin–aldosterone system, plasma bradykinin levels and blood pressure in normotensive postmenopausal women. Geriatr Gerontol Int 2008;8:259–264.
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: Importance of timing of treatment and type of estrogen. Cardiovasc Res.. 2005;66:295–306.
- Dahlöf B. Structural cardiovascular changes in essential hypertension: Studies on the effect of antihypertensive therapy. Thesis, Sweden; 1992. ISBN: 91-628-0592-4.