Abstract
Objective. The aim of the study was to investigate the skin microcirculation blood flow and flowmotion response to heat stress in normotensive subjects with familial predisposition to hypertension and in hypertensive patients. Methods. Normotensives without [NT(−)] or with [NT(+)] familial predisposition and subjects with newly diagnosed hypertension (HT) were studied. Clinic blood pressure (BP) measurements and ambulatory BP monitoring as well as laboratory assessments were performed. Resting (RF), heat (HF) and maximal heat (MHF) blood flows were measured using PeriFlux laser Doppler flowmetry (LDF) and expressed as absolute units (AU) and as index of cutaneous vascular conductance (CVC). Spectral analysis of the skin LDF signal was performed by means of the Perisoft dedicated software. Kruskall–Wallis analysis of variance, χ2 statistic and multivariate reverse regression analysis were used for calculation. Results. The studied population consisted of 70 persons (mean age 36.1±10.3 years, 44.3% women): 17 NT(−), 22 NT(+) and 31 HT, age and gender matched. Higher values of body mass index (BMI), and insulin, glucose and triglyceride levels were observed in HT than in NT groups. RF, HF and MHF were similar in all study groups, but CVC of maximal heat flow differed (p=0.02); in particular, lower values were observed in the HT than in NT(−) group (p=0.01). The study groups differed with regard to total power (p=0.01) and myogenic (p=0.03) origin flowmotion with the lowest values in the NT(+) group. BMI and night BP characteristics were strong predictors of reduction of CVC, MHF and myogenic origin flowmotion. Conclusion. Skin microcirculation response to local heat stress is altered in hypertensive patients with decrease in maximal heat CVC values. Moreover, normotensive subjects with familial predisposition to hypertension are characterized by diminished myogenic origin of skin blood flowmotion.
Introduction
Skin blood flow in humans can increase substantially in response to thermal stress and such reaction represents a vital aspect of normal thermoregulation (Citation1). The thermoregulatory bed of cutaneous microcirculation constitutes about 85%, whereas the nutritive capillary bed contributes up to 15% of the total blood flow (Citation2). Mechanisms for reflex control of thermal skin blood flow include sympathetic adrenergic vasoconstrictor nerves and sympathetic vasodilator nerves, the latter being responsible for 80–90% of the substantial cutaneous vasodilation during whole body heat stress (Citation1). However, local thermal control of skin blood flow includes local adrenergic activity, sensory nerves and nitric oxide. Hyperaemic response provides an integrated index of neurovascular and nitric oxide-dependent cutaneous blood flow regulation (Citation1).
Consistent data show that thermal hyperaemia is impaired in diabetes (Citation3), systemic sclerosis (Citation4) and aging (Citation5). Animal models studies revealed that thermoregulatory vasodilation was also weakened in hypertension (Citation6). Sustained hypertension in men reduced the maximal cutaneous vasodilation induced by local warming of the forearm skin as well (Citation7). Moreover, forearm blood flow during periods of rising internal temperature induced by dynamic exercise was also markedly reduced in hypertensive subjects (Citation8). Nevertheless, the forearm vascular conductance during passive heat stress rose to similar levels in hypertensive and in normotensive subjects, although in normothermia it was lower in hypertensives (Citation9). As a result, the authors suggested that vasodilator tone were greater in hypertensive than in normotensive subjects.
Vessel-to-tissue interactions and exchange of nutrients, gas and heat are to some extent related to vessels vasomotion (Citation10). The absence of flow pulsatility might impair the peripheral perfusion stability and trigger microvascular dysfunction of vital organs (Citation11). Investigation of skin blood flowmotion, using spectral analysis of LDF signal, showed different flowmotion waves of endothelial, sympathetic or myogenic mediated vasomotion origin (Citation12). It has been suggested that changes in rhythms of skin blood flow after incremental tissue loads are mediated by a myogenic control mechanism, and that a metabolic control mechanism acts after heat application to the tissue (Citation13). During normothermia, the forearm skin blood flowmotion among hypertensives showed a post-ischaemic impairment of myogenic and sympathetic components in newly diagnosed patients, and of endothelial and sympathetic components in long-standing patients. (Citation14). Normotensives with a family history of hypertension exhibited increased myogenic microvascular reactivity (Citation15). No similar observations were reported during thermal stress. We decided to estimate the skin microcirculation response to local heat stress among normotensive subjects with familial predisposition to hypertension and in patients with established hypertension.
Patients and methods
Study population
The experimental protocol has been approved by the local Ethics Committee at the Medical College of the Jagiellonian University and it conforms to the guidelines set forth by the Declaration of Helsinki. Subjects were recruited into the study from the ambulatory patients at the hypertensiology centre and from healthy volunteers. Healthy subjects were divided into two groups according to family history of hypertension (one or both parents) as normotensives without [NT(−)] or with [NT(+)] familial predisposition to hypertension. Hypertensive patients (HT) were newly diagnosed and untreated. All participants were free from further known cardiovascular risk factors or related comorbidities. Heavy smokers (more than five cigarettes per day) were excluded from the study. Height and weight were measured, and body mass index (BMI) was calculated.
Blood pressure measurements
Conventional blood pressure (BP) measurements and ambulatory BP monitoring (ABPM) were performed in all study participants. Conventional BP measurements were conducted by the same investigator on the upper right arm with the cuff inflated at heart level using a mercury sphygmomanometer. Before the measurements, the subject remained in the supine position for about 10 min and then underwent three BP measurements at 2-min intervals. Systolic BP (SBP) level was recorded at Korotkoff Phase I and diastolic BP (DBP) at Korotkoff Phase V. Mean values of the two last measurements were used in further analysis. Mean arterial pressure (MAP) was calculated using the formula: MAP=DBP+[SBP–DBP]/3).
ABPM was recorded over a working day using an automatic SpaceLabs 90207 unit. The cuff with the appropriate bladder size was fixed to the studied arm, and the device was set to obtain automatic BP readings at 15-min intervals during the daytime and at 30-min intervals during the night-time. Daytime and night-time were defined using fixed clock time intervals, which ranged from 06:00 to 22:00 h and from 22:00 to 06:00 h, respectively. Investigators performing ABPM were blinded to the clinical status of examined subjects. More than 80% of successful readings were required to qualify the test for subsequent analysis. The mean values and variability (expressed as standard deviation [SD]) of 24-h, daytime and night-time systolic/diastolic BP were analysed.
Laser Doppler flowmetry
All measurements were performed in the morning in a quiet room at temperatures of approximately 21–22°C. Subjects were asked to refrain from smoking and drinking anything containing caffeine at the day of examination. Skin blood flow was measured as cutaneous red blood cell flux using PeriFlux laser Doppler flowmetry (LDF). The LDF probe (Periflux System 5000; Perimed, Järfälla/Stockholm, Sweden) was attached using an adhesive to the right forearm to measure skin blood flow. The recording was performed after a 20-min period of adjustment in supine position, with LDF signal stable for approximately 10 min before the recording.
A 4-min baseline was recorded prior to thermal hyperaemia. Then the local heating units were heated from skin temperature to 44°C over 15–22 s and maintained at this temperature for 8 min (). The day-to-day reproducibility of the thermal hyperaemia has been demonstrated previously (Citation16).
Figure 1. Illustration of compressed resting and thermal flow records. RF, resting flow; HF, heat flow; MHF, maximal heat flow.
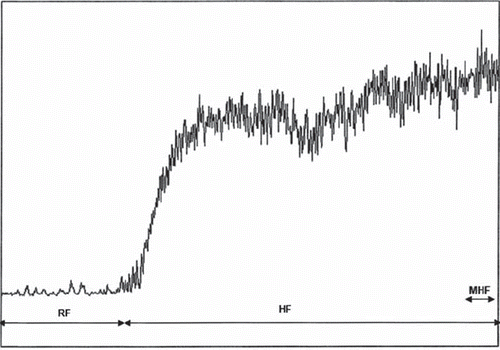
The data were digitized and stored on the computer and analysed off-line with signal processing software (PeriSoft 2.5.5, Perimed). The following data were analysed: mean values of 4 min resting blood flow (RF), 8 min heat flow (HF), 30 s maximal heat flow (MHF).
The values of blood flow were expressed as absolute values (arbitrary units-AU) and as index of cutaneous vascular conductance (CVC) calculated as flux divided by mean arterial pressure (AU/mmHg) (Citation17). Thermal stimulation ratio (TSR) was calculated according to the formula TSR=HF/RF×100%.
Spectral analyses of skin LDF signal of HF was performed using Perisoft dedicated software. This software measures the power spectral density of LDF signal, using the Fast Fourier Transform (FFT) algorithm and a Short Time Fourier Transform (STFT). The detailed data of the chart of frequency analysis report were exported to Microsoft Excel, and the mean values of total power and power of five frequency subintervals were calculated. The total power of the studied interval (0.009–1.6 Hz) and five subintervals related to: (i) endothelium activity (0.009–0.02 Hz); (ii) neurogenic activity (0.02–0.06 Hz); (iii) myogenic activity (0.06–0.2 Hz); (iv) respiration activity (0.2–0.6 Hz) and (v) heart activity (0.6–1.6 Hz) (Citation16) were analysed and expressed as absolute value (AU2/Hz) (Citation12).
Carotid-femoral pulse wave velocity
Pulse wave velocity (PWV) was measured by the same operator, using a Complior device (Colson, France). One transducer was placed at the base of the neck for the common carotid artery and one over the femoral artery. The 10 pulse waves were recorded and stored. The transit time between the carotid and the femoral pulse waves was automatically determined by the software. The distance between the two recording sites was measured. PWV was calculated as the ratio of distance to transit time.
Laboratory investigations
Venous blood samples were obtained from each participant after an overnight fasting (from 06:00 to 08:00 h) for the determination of blood count, serum glucose, urea (BUN) and sodium levels and lipid profile [total cholesterol, triglyceride, low-density (LDL) and high-density lipoprotein (HDL) levels], according to established methods. Plasma osmolarity [mosm/l] was calculated according to the formula: 2[Na+]+[glucose]+[BUN] in mmol/l.
Part of the blood specimen was centrifuged and stored at −80°C for subsequent analyses of insulin and endothelin levels. Insulin (μU/ml) was measured using electrochemiluminescence immunoassay (Roche Elecsys 2010), and endothelin (pg/ml) using immunosorbent assay (ELISA, Parameter, R&D Systems).
Statistical analysis
Results were expressed as means±SD. Between-group comparisons were made using Kruskall–Wallis analysis of variance for non-parametric data with post hoc analysis to identify individual differences between groups, and using the χ2 test to investigate differences within the distribution of categorical variables. Spearman correlation coefficients were calculated for all variables in the entire study population. Multivariate reverse regression analysis was used to detect the parameters of the study population, which can constitute predictors of CVC MHF and myogenic activity of skin flowmotion. The simplest significant model was selected. A p-value < 0.05 was considered statistically significant.
Results
The study population consisted of 70 persons (mean age-36.1±10.3 years, 44.3% women): 17 NT(−), 22 NT(+) and 31 HT, age and gender matched. Groups differed according to the BMI, glucose, triglyceride and insulin levels (), as well BP values (). BP values were significantly higher in hypertensives as opposed to normotensive groups (data of post hoc analysis were not shown). Variability of 24-h BP was similar among studied groups, although variability of SBP during day and night and variability of diurnal DBP were lower in NT(+) than in NT(−).
Table I. Characteristics of the study groups.
Table II. Clinic and ambulatory blood pressure (BP) values in the study groups.
The mean values of RF and HF were similar in all studied groups, both absolute values and CVC (). TSR was also comparable. However, MHF revealed the higher values among NT(−) than in others. CVC of MHF was the lowest among hypertensives, especially compared with the NT(−) group.
Table III. Characteristics of resting and thermal flows in study groups.
The results of the spectral analysis of skin LDF signal have been demonstrated in . The total power spectrum of heat flowmotion was the lowest in NT(+) with the strongest differences compared with NT(−) (p=0.01). The lowest power of myogenic origin was observed in NT(+) with the strongest differences compared with NT(−) (p=0.02). Power of respiration and heart activity behaved in the same way as the power of myogenic activity.
Table IV. Power spectral density values of the different skin blood flowmotion frequency intervals in thermal condition among the study groups.
Univariate analysis revealed significant negative correlations between the myogenic origin of flowmotion and haematocrit (r=–0.32), male gender (r=–0.36), and positive with HDL-cholesterol (r=0.33). Multiple regression models included age, gender, BMI, PWV, values of clinic BP and ABPM measurements, blood count, osmolarity, fasting blood glucose, lipids, insulin, and endothelin levels as independent variables. Significant predictors of CVC MHF and myogenic activity have been presented in . BMI, clinic DBP and variability of night diastolic BP were significant predictors of CVC MHF reduction. The minor power of myogenic flowmotion might be expected when BMI and night systolic BP are increased.
Table V. Significant predictors of cutaneous vascular conductance of maximal heat flow and myogenic origin of skin blood flowmotion in multiple regression models.
Discussion
Local thermal reaction was diminished among patients with untreated hypertension and normotensives with parental history of hypertension, compared with normotensives without family predisposition. Hypertensives revealed CVC of MHF reduction, and NT(+) subjects showed decline of total power spectrum of heat flowmotion and of myogenic origin than NT(−) group. Predictors of CVC MHF reduction were BMI, variability of night diastolic BP and clinic values DBP. BMI and night systolic BP were also constituted predictors of myogenic origin flowmotion decline.
The degree of vasodilation induced by local skin warming has been proposed as a clinical tool for evaluation of vasomotor dysfunction in diabetes and other disease states (Citation7,Citation18). Local human skin warming causes a biphasic substantial vasodilation (Citation1,Citation9). There is an initial rapid increase in blood flow during the first minutes, then a moderate decrease followed by a slower vasodilation that attains a plateau. The initial rapid-phase vasodilation relies predominantly on local activity of sensory nerves, primarily C-fibre afferents, which when stimulated, cause localized vasodilation via antidromic release of calcitonin gene-related peptide, substance P and neurokinin A. It has been demonstrated that nitric oxide (NO) generation by NO syntase plays a major role in the vasodilation induced by prolonged local warming (Citation9).
The lower values of CVC MHF in the hypertensives group than in the others may indicate that the studied patients presented worse endothelial function. It is generally approved that hypertension is accompanied by endothelial dysfunction, in which oxidant generation and uncoupling of endothelial NO syntase play an important role (Citation19,Citation20). The differences of maximal heat CVC between NT and HT groups may also indicate the structural changes in the skin vasculature caused by rarefaction, vascular hypertrophy, or both. Significant structural rarefaction of skin capillaries has been reported in patients with borderline intermittent primary hypertension (Citation21) and in normotensive individuals with familial predisposition to hypertension (Citation22,Citation23). Remodelling of arterial wall plays important role in the vascular resistance increase (Citation24,Citation25).
The disturbance of neurogenic response of local sensory nerves may constitute a further possible mechanism of decrease of CVC MHF. It has been lately presented that neurogenic flare response to nociceptive stimuli during skin heating registered by laser Doppler imager clearly detected loss of C-fibre function in neuropathic subjects with type 2 diabetes before its detection by other currently available methods (Citation26). Neurogenic nociceptor-mediated vasodilation is impaired in subjects with type 2 diabetes, even though endothelial and sympathetic function are relatively intact (Citation27). It is suggested that heat-induced vasodilation may be a specific test of small heat-sensitive C-fibre peripheral neurons and can constitute an integral part of the metabolic syndrome, and so also in many patients with hypertension (Citation27,Citation28). The results of our research have not revealed any differences in neurogenic activity frequency band flowmotion. Nevertheless, our study population was relatively healthy, without diabetes or metabolic syndrome.
Usually, compared with preheating blood flow, heat stress causes decreased flowmotion of myogenic origin in the heating and post-heating periods (Citation13,Citation29). The decreased myogenic activity is connected with mild inflammatory reaction produced by heat. Formed locally, chemical mediators act to decrease the smooth muscle tone and increase capillary and post-capillary venule permeability, resulting in vasodilation of resistance vessels (Citation30). The studied hypertensives and normotensives with familial predisposition to hypertension presented greater diminishing of myogenic activity than normotensives without family history. That might suggest that such a mechanism is disturbed among hypertensives and subjects with familial predisposition to hypertension, probably connected to a higher inflammatory reaction.
Alongside BP characteristics, it was BMI that constituted a strong predictor both of CVC MHF decrease and myogenic activity of flowmotion reduction. Human obesity is associated with increased heat production; however, the subcutaneous adipose tissue provides an insulating layer that impedes heat loss (Citation31,Citation32). In addition, obese subjects with or without hyperglycaemia and/or hyperinsulinaemia demonstrated impairment of small fibre neuronal function (Citation33). Results of our study confirmed that a significant association can be found between BMI and local skin reactivity to thermal stress, even without overweight and obesity.
Our study has certain limitations. The number of studied subjects with or without familial predisposition to hypertension was relatively small. We also cannot prove that flowmotion of skin microcirculation is similar to that from the other organs. Moreover, the approved protocol of LDF signal registration made separate analysis of heat flow phases difficult.
Nevertheless, the results of our investigation for the first time exhibited that not only patients with advanced stages of hypertension and but also those with early ones and even normotensive subjects with familial predisposition to hypertension presented alterations in microcirculations response to thermal stress. Hypertensives revealed lower values of maximal heat CVC than the NT(−) group. Normotensives with parental history of hypertension showed the disturbance of myogenic origin flowmotion. BMI and night BP characteristics constitute strong predictors of CVC MHF and myogenic origin of skin blood flowmotion reduction. However, further studies are needed to explain precise mechanisms of our observations.
Acknowledgements
The research was supported by the Ministry of Science and Higher Education within the Contract No. 4P05B123.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Charkoudian N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612.
- Pries AR. Physiology of microcirculation and organ perfusion. Levy BI, Struijker-Boudier HAJ. Role of micro and macrocirculation in target organ damage in diabetes and hypertension. 1st. Oxford: Blackwell Publishing Ltd; 2009. 14–30.
- Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, . Delayed threshold for active cutaneous vasodilation in patients with type 2 diabetes mellitus. J Appl Physiol. 2006;100:637–641.
- Roustit M, Simmons GH, Carpentier P, Cracowski JL. Abnormal digital neurovascular response to local heating in systemic sclerosis. Rheumatology. 2008;47:860–864.
- Pierzga JM, Frymoyer A, Kenney WL. Delayed distribution of active vasodilation and altered vascular conductance in aged skin. J Appl Physiol. 2003;94:1045–1053.
- O'Leary DS, Wang G. Impaired thermoregulatory cutaneous vasodilation in spontaneously hypertensive rats. J Appl Physiol. 1994;77:692–696.
- Carberry PA, Shepherd AMM, Johnson JM. Resting and maximal forearm SkBF are reduced in hypertension. Hypertension. 1992;20:349–355.
- Kenney WL, Kamon E. Comparative physiological responses of normotensive and essentially hypertensive men to exercise in the heat. Eur J Appl Physiol. 1984;52:196–201.
- Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829.
- Fullerton A, Stűcker M, Wilhelm K-P, Wardell K, Anderson C, Fischer T, . Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. Contact Dermatitis. 2002;46:129–140
- Vasků J, Wotke J, Dobsák P, Baba A, Rejthar A, Kuchtícková S, . Acute and chronic consequences of non-pulsatile blood flow pattern in long-term total artificial heart experiment. Pathophysiology. 2007;14:87–95.
- Stefanovska A, Bracčicč M, Kvernmo HD. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng. 1999;46: 1230–1239.
- Brienza DM, Geyer MJ, Jan YK. A comparison of changes in rhythms of sacral skin blood flow in response to heating and indentation. Arch Phys Med Rehabil. 2005;86:1245–1251.
- Rossi M, Carpi A, Di Maria C, Galetta F, Santoro G. Spectral analysis of laser Doppler skin blood flow oscillations in human essential arterial hypertension. Microvasc Res. 2006; 72:34–41.
- Maver J, Strucl M, Accetto R. Autonomic nervous system and microvascular alterations in normotensives with a family history of hypertension. Blood Press. 2004;13:95–100.
- Grodzicki T, Necki M, Cwynar M, Gryglewska B. Laser Doppler flowmetry – Repeatability of the method. Przegl Lek. 2003;60:89–91 (Polish).
- Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, . Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction. J Physiol. 2007;585:627–634.
- Sandeman DD, Pym CA, Green EM, Seamark C, Shore AC, Tooke JE. Microvascular vasodilatation in feet of newly diagnosed non-insulin dependent diabetic patients. BMJ. 1991; 302:1122–1123.
- Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: A new target for treatment? Circulation. 2001;104:735–740.
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992.
- Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658.
- Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, . Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–1879.
- Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–178.
- Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21: 391–397.
- Mulvany MJ. Structural abnormalities of the resistance vasculature in hypertension. J Vasc Res. 2003;40:558–560.
- Krishnan ST, Rayman G. The LDIflare: A novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. 2004;27:2930–2935.
- Stansberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI. Primary nociceptive afferents mediate the blood flow dysfunction in non-glabrous (hairy) skin of type 2 diabetes: A new model for the pathogenesis of microvascular dysfunction. Diabetes Care. 1999;22:1549–1554.
- Vinik A, Erbas T, Stansberry K, Pittenger G. Small fiber neuropathy and neurovascular disturbances in diabetes mellitus. Exp Clin Endocrinol Diabetes. 2001;109:451–473.
- Geyer MJ, Jan YK, Brienza DM, Boninger ML. Using wavelet analysis to characterize the thermoregulatory mechanisms of sacral skin blood flow. J Rehabil Res Dev. 2004;41: 797–806.
- Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia, PA: Elsevier; 2006. 889–901.
- Kasai T, Hirose M, Matsukawa T, Takamata A, Tanaka Y. The vasoconstriction threshold is increased in obese patients during general anaesthesia. Acta Anaesthesiol Scand. 2003;47: 588–592.
- Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. 2006;55: 515–524.
- Herman RM, Brower1 JB, Stoddard DG, Casano AR, Targovnik JH, Herman JH, . Prevalence of somatic small fiber neuropathy in obesity. Intern J Obes. 2007;31: 226–235.