Abstract
Background and aim. The prevalence of left ventricular hypertrophy (LVH) in human hypertension has been mostly documented in population-based samples and selected hypertensive cohorts. Rather scant data are available from clinical practice. Thus, we examined the prevalence of LVH in a large group of hypertensive patients referred by general practitioners to a routine echocardiographic examination. Methods. A total of 2249 hypertensive subjects (mean age 62 years, 52.3% men, 84.5% treated) referred by their practitioners to 17 outpatient echocardiographic laboratories across Italy for detection of hypertensive early cardiac damage were included in the study. LVH was defined as left ventricular mass (A) ≥ 225/163 g, (B) ≥ 116/96 g/m2, (C) ≥ 49/45 g/m2.7 in men/women, respectively; LVH was graded as mild, moderate and severe according to Lang's report. Results. Overall, patients with LVH were 58%, 58% and 65% by criteria A, B and C, respectively. LVH was mild in 33% (A), 36% (B) and 29% (C), moderate in 31% (A), 28% (B) and 27% (C), and severe in 36% (A), 36% (B) and 44% (C). Conclusions. Data provided by this multicentre nationwide survey support the view that, despite therapeutic interventions, LVH remains a highly frequent phenotype in human hypertension and that severe LVH is present in a large fraction of hypertensives.
Introduction
Left ventricular hypertrophy (LVH) is a preclinical organ damage frequently observed in human hypertension, reflecting the impact of chronic pressure overload on the heart (Citation1). Additional haemodynamic and non-haemodynamic factors advocated in the pathogenesis of this cardiac phenotype include volume overload, genetic, racial and hormonal factors (Citation2–4). Overall, LVH in hypertensive patients is a marker of the burden on the heart of multiple modifiable and non-modifiable risk factors.
LVH, as assessed by electrocardiography or more accurately by echocardiography, is a strong predictor of cardiovascular (CV) morbidity and mortality. In hypertensive cohorts and other clinical conditions, LVH has been documented to be independent of conventional risk factors, including ethnicity, gender and age (Citation5–11). Prospective studies have consistently shown that effective long-term blood pressure (BP) lowering may prevent or reduce LVH (Citation12). Moreover, a persistently normal left ventricular (LV) mass (LVM) or LVH regression are both conditions associated to a lower risk of incident CV disease compared with new developed or persistent LVH (Citation13–17). Based on such evidence, a search for LVH is recommended in the initial assessment as well as in the follow-up of hypertensive patients.
The prevalence rates of LVH as assessed by echocardiography markedly vary among studies, ranging from 3% to 77%, depending on clinical characteristics of the population study and diagnostic criteria applied (Citation18,Citation19). Available data on LVH prevalence mostly derive from population-based studies and selected hypertensive cohorts. Rather scant data are available from surveys conducted in clinical practice.
Therefore, we sought to investigate this issue in a survey involving a large number of hypertensive patients referred by their general practitioners to nationwide echocardiographic laboratories for a routine examination for detection of hypertensive cardiac damage. Our primary aim was to determine LVH prevalence and severity.
Materials and methods
For the present analysis, data derived from two Italian multicentre surveys performed by the Working Group on Heart and Hypertension of the Italian Society of Hypertension during the period 2008–2009 have been pooled.
Briefly, the first study, including 2646 patients enrolled from 14 centres, was designed to assess how frequently an echo exam is requested in current practice in order to detect hypertensive subclinical cardiac damage (Citation20).
The second study, including 2513 patients enrolled from nine centres, was undertaken to investigate the difference between self-reported and measured weight and height in individuals referred to an outpatient echo-lab by general practitioners for a routine examination and the impact of the difference in these anthropometric parameters on the estimated prevalence of LVH.
In both studies, participating laboratories were requested to enrol at least 100 outpatients of either sex, older than 18 years of age, consecutively referred to laboratories by their general practitioners, whose written prescription identified the clinical indications for the examination. No exclusion criteria were defined for the enrolment with the exception of patients with altered LV geometry such as to cause an unreliable LVM estimation. Patients’ demographic data, including self-reported weight and height, medical history and medications were collected in a structured questionnaire by attending physicians at echo-labs.
Of the 17 units in these surveys, 10 served as outpatient echo-labs within university hypertension clinics (seven of internal medicine and three of clinical cardiology) and the remaining seven within hospital cardiology units.
Measurements
Clinic BP had to be measured with a mercury sphygmomanometer using an appropriately sized cuff; measurements were performed in the echocardiographic laboratories after the subjects had rested for 3–5 min in the sitting position. Three measurements were taken from the non-dominant arm at 1-min intervals and the average was used to define patient's representative values.
Echocardiographic procedures
Echo and Doppler examinations were performed in each participating centre according to a standardized protocol. In brief, M-mode, two-dimensional and Doppler echo examinations were carried out by high performance instruments equipped with 2–2.5-MHz imaging transducers. In particular, end-diastolic (d) and end-systolic (s) LV internal diameters (LVID), interventricular septum thickness (IVST) and posterior wall thickness (PWT) were measured from two-dimensionally guided M-mode tracings recorded at a speed of 50–100 cm/s, during 3–5 consecutive cycles according to the Penn convention. Relative wall thickness (RWT) was defined by the ratio of PWT plus IVST to LVIDd. LVM was estimated by Devereux's formula {1.04[(IVSTd+ PWTd +LVIDd)3−LVIDd3]−13.6} (Citation21) and normalized to BSA or height2.7 (h2.7). LV ejection fraction was measured from the four-chamber apical projection by the area product × ventricular length.
Definition of LVH and LV geometric patterns
LVH was defined by absolute and normalized LVM according to the following gender-specific thresholds: (A) LVM ≥ 225/163 g; (B) LV mass index (LVMI) ≥ 116/96 g/m2; (C) LVMI ≥ 49/45 g/h2.7 (Citation22) in men/women, respectively.
The values of LVM and LVMI were graded as mildly abnormal [(A) 225–258 g in men, 163–186 g in women; (B) 116–131 g/m2 in men, 96–108 g/m2 in women; (C) 49–55 g/h2.7 in men, 45–51 g/h2.7 in women], moderately abnormal [(A) 259–292 g in men, 187–210 g in women; (B) 132–148 g/m2 in men, 109–121 g/m2 in women; (C) 56–63 g/h2.7 in men, 52–58 g/h2.7 in women] and severely abnormal [(A) ≥ 293 g in men, ≥ 211 g in women; (B) ≥ 149 g/m2 in men and ≥ 122 g/m2 in women; (C) ≥ 64 g/h2.7 in men, ≥ 59 g/h2.7 in women] (Citation22).
Patterns of abnormal LV geometry were defined as follows: (i) LV concentric remodelling (normal LVMI combined with RWT≥0.43); (ii) eccentric LVH (increased LVMI combined with RWT < 0.43); (iii) concentric LVH (increased LVMI combined with RWT ≥ 0.43 (Citation22,Citation23).
Four more gender-specific LVH diagnostic criteria have been considered in additional analyses: (D) LVM/height ≥ 127/100 g/m (Citation22); (E) IVST or PWT ≥ 1.1/1.0 cm (Citation22); (F) IVST and PWT ≥ 1.1/1.0 cm (Citation22); (G) LVMI ≥ 125/110 g/m2 in men/women, respectively (Citation23).
Two files per patient have been e-mailed to the Clinical Research Center, Istituto Auxologico Italiano, University of Milano-Bicocca, acting as coordinating centre for the final analysis including: (i) a questionnaire containing demographic and clinical data, and (ii) an echo diagnostic report.
The protocols of both studies were approved by the Ethics Committee of the coordinating centre (Istituto Auxologico Italiano and University of Milano-Bicocca).
Statistical analysis
Statistical analysis was performed by the SAS System (version 6.12; SAS Institute Inc., Cary, NC, USA) and was mostly descriptive; values were expressed as means ± SD or as percentages. Mean values have been compared by Student's t-test for independent samples and categorical data analysed by the chi-square test or the Fischer's exact test when appropriate. The strength of correlation between variables was tested by linear correlation analysis and multiple regression analysis. The value of p < 0.05 was considered statistically significant.
Results
From a total of 5129 participants, 2299 hypertensive patients were identified and recruited in both surveys by 17 participating centres between February 2008 and June 2009; 50 of these patients were excluded because of unavailable or incomplete echocardiographic reports. Thus, 2249 subjects (52.3% males) were eligible for the final analysis; their clinical characteristics are reported in . Briefly, mean age was 62 ± 13 years, mean BP 140 ± 17/83 ± 10 mmHg, 84.5% of the study sample was on antihypertensive drugs, 26.4% was obese according to the 1998 National Institutes of Health classification (i.e. BMI ≥ 30 kg/m2), 8.2% was affected by type 2 diabetes mellitus, according to revised American Diabetes Association criteria (fasting serum glucose level ≥ 6.99 mmol/l and/or on oral hypoglycaemic agent and/or insulin). Women were older, had higher systolic (SBP) and lower diastolic BP (DBP), higher prevalence of obesity and diabetes than their counterparts.
Table I. Clinical characteristics of the study population as a whole and divided by gender.
Prevalence, type and severity of LVH
Overall, 1307 patients (58.1%) were found to have LVH by criterion A (absolute LVM), 1311 (58.2%) by criterion B (LVM/BSA) and 1460 (64.9%) by criterion C (LVM/height2.7), according to the gender-specific thresholds indicated under Methods (Citation22).
As for LV geometric patterns, eccentric and concentric LVH had a similar prevalence regardless the criteria used (A: 28.5% vs 29.0%; B: 29.4% vs 29.2%; C: 32.2% vs 33.0%, respectively, p=ns).
Echocardiographic data shown in indicate that LVIDd, LVIDs, septal and PWT, absolute and indexed LVM, left atrial and aortic root diameter were significantly higher in men than women, whereas LV relative wall thickness, mitral E and A velocity, E/A ratio and prevalence rates of LVH, regardless of the criteria used, showed an opposite trend.
Table II. Echocardiographic findings of the study population as a whole and divided by gender.
Severe LVH, defined according to the three primary criteria, was found in a significant portion of the study population, ranging from 20.8% (LVM/BSA) to 28.9% (LVM/height2.7).
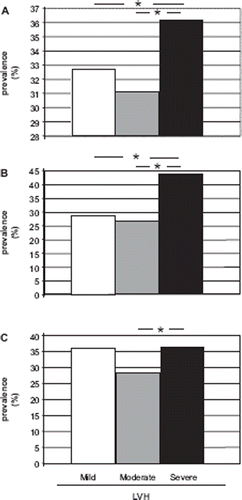
shows that LVM was severely increased in more than one third of patients with LVH (range 36%–44%). Notably, severe LVH represented the most frequent alteration among women, ranging from 41.2% (LVM/BSA) to 49.5% (LVM/height2.7); prevalence of severe LVH was much lower among men, ranging from 15.8% (LVM/BSA) to 39.5% (LVM/height2.7), p<0.01.
Figure 1. Prevalence rates of mild, moderate and severe left ventricular hypertrophy in the whole hypertensive study population (n=2249) according to three diagnostic criteria: (A) left ventricular mass ≥225/163 g; (B) left ventricular mass index ≥116/96 g/m2; (C) left ventricular mass index ≥49/45 g/h2.7 in men and women; *p at least <0.05.
Prevalence and severity of LVH: additional analyses
reports prevalence rates of LVH according to additional criteria as specified in the Methods. In the entire sample, LVH prevalence ranged from 32.6% (LVM index >125/110 g/m2) to 64.4% (IVST ≥1.1/1.0 cm in men/women). Again, LVH was more prevalent in women according to all but one criteria (LVM index >125/110 g/m2).
Table III. Prevalence rates of left ventricular hypertrophy according to five additional criteria in the study population as a whole and divided by gender.
As for LVH severity, about 40% of patients with LVH defined by LVM/height, had a severe degree of LVH (35.6% men and 46.8% women), whereas a minimal fraction of the patients (<0.1%) fulfilled the criteria of severity of IVST and PWT.
As 2007 ESH/ESC guidelines (Citation23) do not provide thresholds for defining LVH severity, such a categorization was not performed in patients with LVH according to ESH/ESC criteria.
LVH, antihypertensive treatment and BP control
Among the 1900 patients (51.5% men) on antihypertensive medication, clinic BP was controlled (i.e. <140/90 mmHg) in 39.2% of cases (39.9% in men and 38.4% in women). reports prevalence rates of LVH, according to the gender-specific criteria recommended by both inter-society Lang's report (Citation22) and ESH/ESC guidelines (Citation23). A progressive increase in LVH occurred from untreated hypertensives, treated patients with satisfactory BP control and treated hypertensives with uncontrolled BP.
Table IV. Prevalence rates of left ventricular hypertrophy according to five criteria in untreated hypertensives (UNTH), in treated hypertensives with (THa) and without blood pressure control (THb).
Correlation analyses
The relation of absolute LVM with clinical variables such as age, gender, weight, height, SBP, DBP, heart rate, anti-hypertensive treatment and diabetes mellitus was analysed by a stepwise multivariable regression analysis. Among these variables, weight (β = 0.350), male gender (β = 0.287), age (β = 0.199), followed by SBP (β = 0.129) were in ranking order the best correlates of LVM (p < 0.0001 for all).
Discussion
The present study assessed the prevalence and severity of LVH in a large cohort of hypertensive individuals referred by their practitioners to outpatient echo-labs for evaluation of hypertension-related subclinical cardiac damage. To our knowledge, this is the first time that such an aspect has been addressed. LVH was defined according to three gender-specific criteria endorsed by the Lang's inter-society report (Citation22), based on unadjusted LVM as well as on LVM normalized to either BSA or height to the allometric power of 2.7. The prevalence of LVH in our population was quite high, ranging from 58% to 65%. Our survey was also focused on the degree of LVH and showed that more than one third of patients with LVH had a severely increased absolute or indexed LVM, regardless of the criteria used.
Several aspects of our findings need comment.
Estimates of LVH depend not only on clinical characteristics of subjects under investigation but also on the criteria used to define this cardiac phenotype. Indeed, additional analyses of our series based on more restrictive criteria recommended by 2007 ESH/ESC guidelines (Citation23), reduced LVH prevalence to 33%. As previously discussed, available studies examining the prevalence and clinical correlates of LVH mostly refer to selected hypertensive patients and to the general population. Nonetheless, data from two studies performed in primary care settings may be usefully compared with our results. In the VITAE study (Ventriculo Izquierdo Tension Arterial Espana), including 946 essential hypertensives seen in 39 primary care centres, LVH prevalence ranged from 59%, according to the sex-specific criterion of LVM index > 111/102 g/m2, to 73% according to LVM index > 50/47 g/m2.7 (Citation24). Similar figures (52–72%) have been reported by Conrady et al. among 734 hypertensives from primary care outpatient clinics examined in the St Petersburg study (Citation25).
In the present study population, all diagnostic criteria but one (i.e. 125/110 g/m2), resulted in a substantially higher LVH prevalence in women.
This gender-related difference is in line with findings reported by Levy et al. in the Framingham population (Citation26), by de Simone et al. in the Strong Heart Study (Citation27) and by Wachtell et al. in the LIFE cohort (Citation19). Nonetheless, the relationship between LVH and gender remains a controversial issue. Several studies, indeed, failed to observe any difference between genders (Citation27) or showed that gender-related LVH differences are highly dependent on the echocardiographic criteria and methods chosen for LVM normalization to body size (Citation24,Citation25).
The present study, to our knowledge, is the first one providing some information upon the severity of hypertensive subclinical cardiac damage in clinical practice: in a large fraction (>30%) of patients with LVH, indeed, a severe increase in LVM has been documented. Again, severe LVH was significantly more frequent in women than in men and the difference was independent from the criteria used for grading this adverse phenotype. A growing body of evidence in the hypertensive setting supports a greater likelihood of LVH in women. Recently, Okin et al. demonstrated that regression of electrocardiographic LVH in response to long-term antihypertensive therapy is less pronounced in women than in men, even after adjustment for other factors affecting LVH regression (Citation28).
These observations may contrast with gender differences in biochemical and morphological adaptations of the heart to haemodynamic overload reported in both experimental and human studies. Luchner et al. demonstrated that LVH development in response to similar increments in SBP was more rapid and severe in men than in women (Citation29).
In the present survey, a marked disagreement in detecting severe LVH was observed between the criteria based on LVM estimates and those based on LV wall thickness. This latter approach, indeed, indentified a severe hypertensive-related cardiac involvement only in a minimal fraction of patients. This suggests that LVH grading based on LV thickness systematically underestimates advanced cases of subclinical cardiac damage.
The relationship between LVH prevalence, antihypertensive treatment and BP control was investigated in a population-based survey in a pioneering paper by Mancia et al. (Citation30). In that study, a stepwise increase in LVH occurred from normotensives (4%), untreated subjects with recently discovered hypertension (14%), treated hypertensives with good BP control (19%) and treated uncontrolled hypertensives (29%). Our data extend the notion to a different setting, by showing that hypertension-related cardiac structural abnormalities are less frequent in treated hypertensives with BP control than in patients with inadequate BP control or in never-treated hypertensives. Moreover, our results emphasize the role of BP lowering interventions in LVH prevention and regression.
Concentric LV geometry is considered a response to pressure overload aimed at reducing wall stress. Volume overload, on the other hand, may also play a key role in LV adaptations in hypertension and explain the high prevalence of eccentric LVH reported in numerous studies (Citation19,Citation30,Citation31). In our series, at variance from previous reports, a similar incidence of concentric and eccentric LVH has been observed. It should also be pointed out that echocardiographic concentric LV remodelling (i.e. normal LVM with increased relative wall thickness) was present in a noticeable portion of our patients (ranging from 9% to 13%); thus, concentric LV geometry was probably much higher than eccentric. Several factors may explain the high prevalence of concentric LVH in our study: (i) old age (approximately half of the patients were elderly); (ii) duration of hypertension (only 15% of patients were untreated, i.e. in the initial phase); (iii) up-dated and less restrictive criteria used to define LV concentric geometry compared with previous studies.
Some limitations of our study deserve to be mentioned. First, extrapolation of results to the general population of hypertensives may be inappropriate, as our data derive from a subset of patients referred to echo-labs for evaluation of subclinical cardiac damage. Second, the lack of a centralized reading centre for echo examinations may have reduced the accuracy of LVH detection. It should be pointed out, however, that a study with practical implications, such as the present one, should mostly rely on diagnostic abilities reflecting the “real word”. Third, we are aware that BP control assessed by a single visit rather than repeated in office and out-of-office measurements, is a rough estimate of effective BP lowering interventions.
In conclusion, data provided by this multicentre nationwide survey strongly support the view that LVH remains a highly frequent phenotype in human hypertension despite therapeutic interventions and that LVM is severely increased in a large fraction of patients with LVH. This implies that echocardiography will contribute to reduce the burden of CV diseases in hypertensive patients with LVH only when more aggressive non-pharmacological and pharmacological interventions are systematically implemented by general practitioners.
Appendix: List of investigators
Download PDF (32.5 KB)Conflict of interest: None.
References
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603.
- Schmieder RE. The role on non-haemodynamic factors in the genesis of LVH. Nephron Dial Transplant. 2005;20:2610–2612.
- Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin–angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219.
- Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500–508.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, . Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–390.
- Larsen CT, Dahlin J, Blackburn H, Scharling H, Appleyard M, Sigurd B, . Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave; the Copenhagen City Heart Study. Eur Heart J. 2002;23:315–324.
- Lonn E, Mathew J, Pogue J, Johnstone D, Danise K, Bosch J, . Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high risk patients. Eur J Cardiovasc Prev Rehabil. 2003;10:420–428.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352.
- Cipriano C, Gosse P, Bemurat L, Mas D, Lemetayer P, N'Tela G, Clementy J. Prognostic value of left ventricular mass and its evolution during treatment in the Bordeaux cohort of hypertensive patients. Am J Hypertens. 2001;14:524–529.
- Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–963.
- Bombelli M, Facchetti R, Carugo S, Madotto F, Arenare F, Quarti-Trevano F, . Left ventricular hypertrophy increases cardiovascular risk independently on in-office or out-of office blood pressure values. J Hypertens. 2009;27:2458–2564.
- Fagard RH, Celis H, Thijs L, Woulters S. Regression of left ventricular mass by antihypertensive treatment: A meta-analysis of randomized comparative trials. Hypertension. 2009;54:1084–1091.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, . Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54.
- Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, . Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621.
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, . Left ventricular concentric geometry during treatment negatively affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738.
- Fagard RH, Staessen JA, Thijs L, Celis H, Birkenhäger WH, Bulpitt CJ, . Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Prognostic significance of electrocardiographic voltages and their serial changes in elderly with systolic hypertension. Hypertension. 2004;44:459–464.
- Scott DS, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, . Effect of angiotensin recetor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: A randomized. Lancet. 2007;369:2079–2087.
- Wachtell K, Bella JN, Liebson PR, Gerdts E, Dahlof B, Aalto T, . Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population. The LIFE Study. Hypertension. 2000;35:6–12.
- Cuspidi C, Negri F, Giudici V, Capra A, Muiesan ML, Agabiti-Rosei E, . Echocardiography in clinical practice: The burden of arterial hypertension. A multicenter Italian Survey. J Hum Hypertens. 2009; Nov 12. [Epub ahead of print].
- Devereux RB, Reickek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- 2007 Guidelines for the Management of Arterial Hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Coca A, Gabriel R, de la Figuera M, Lopez-Sendon JL, Fernandez R, Sagastagoitia JD, . On behalf the VITAE Investigators. The impact of different echocardiographic diagnostic criteria on the prevalence of left ventricular hypertrophy in essential hypertension. J Hypertens. 1999;17:1471–1480.
- Conrady AO, Rudomanov OG, Zaharov DV, Krutikov AN, Vahrameeva NV, Yakovleva OI, . Prevalence and determinants of left ventricular hypertrophy and remodeling patterns in hypertensive patients: The St. Petersburg Study. Blood Press. 2004;13:101–109.
- Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: The Framingham Heart Study. Am J Cardiol. 1987;59:956–960.
- de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, . For the Strong Heart Study Investigators. Normalization for body size and population attributable risk of left ventricular hypertrophy. Am J Hypertens. 2005;18:191–196.
- Okin PM, Gerdts E, Kjeldsen SV, Julius S, Edelman JM, Daholf B, Devereux RB, for the LIFE Investigators. Hypertension. 2008;52:100–106.
- Luchner A, Brockel U, Muscholl M, Hense HW, Doring A, Riegger GAJ, Schunkert H. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: A population-based study. Cardiovasc Res. 2002;53:720–727.
- Mancia G, Carugo S, Grassi G, Lanzarotti A, Schiavina R, Cesana G, Sega R. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control. Data from the PAMELA population. Hypertension. 2002;39:744–749.
- Friedrich N, Aumann N, Dorr M, Felix SB, Nauck M, Wallaschofski H, Volzke H. Lack of association between insulin-like growth factor-1 or insulin-like growth factor-binding protein-3 and left ventricular hypertrophy: Results of the Study of Health in Pomerania. J Hypertens. 2009; Dec 29. [Epub ahead of print].