Abstract
Background. Significant numbers of asymptomatic hypertensive patients are attacked by subclinical target organ damage (TOD) such as proteinuria, left ventricular hypertrophy and carotid atherosclerosis. Platelets become activated in uncontrolled hypertension and play a crucial role in increased thrombotic tendency. Mean platelet volume (MPV) is one of the markers that correlate closely with platelet activity. We aimed to investigate the relationship between MPV levels and subclinical TOD in newly diagnosed hypertensive patients. Methods. 80 newly diagnosed hypertensive patients were enrolled to this cross-sectional study. Ambulatory blood pressure monitoring was performed for all patients. Left ventricular mass index (LVMI), carotid intima-media thickness (IMT) and urine albumin/creatinine ratio (UACR) were measured as indices of cardiac, vascular and renal damage, respectively. MPV was measured from blood samples collected in EDTA tubes and high-sensitivity C reactive protein (hs-CRP) was measured by using nephlometer. Results. MPV was significantly correlated with 24-h systolic–diastolic blood pressure (r = 0.52 and r = 0.55, respectively). Correlation analysis indicated that MPV was moderately related with UACR, LVMI, carotid IMT and hs-CRP (r = 0.50, r = 0.55, r = 0.60 and r = 0.69, respectively, p = 0.0001). Multivariable analysis identified that MPV levels were independently associated with severity of proteinuria, carotid IMT and LVMI (p = 0.001). Conclusion. Our findings suggested that MPV levels were associated with severity of subclinical TOD including; carotid atherosclerosis, left ventricular hypertrophy and renal damage, in hypertensive patients. In addition to this, MPV levels were significantly correlated with hs-CRP levels and 24-h ambulatory blood pressure measurements.
Introduction
Previous studies have shown increased platelet activity and size in essential hypertension (Citation1,Citation2). There is increasing evidence that platelets become activated especially in patients with uncontrolled hypertension (Citation3,Citation4). The high blood pressure leads to changes to the endothelium and platelets as well as in an abnormal increase in the levels of prothrombotic substances that affect the coagulation and fibrinolytic pathways. These abnormalities in hypertension can be related to target organ damage (TOD), prognosis and treatment (Citation5,Citation6).
Mean platelet volume (MPV), the most commonly used measure of platelet size, is a potential marker of platelet reactivity. Larger platelets are metabolically and enzymatically more active, and has greater prothrombotic potential (Citation7). Elevated MPV is associated with other markers of platelet activity, including increased platelet aggregation, thromboxane synthesis and b-thromboglobulin release, and increased expression of adhesion molecules (Citation8,Citation9). We have shown increased MPV levels in hypertensive patients compared with healthy subjects in our previous study (Citation10).
Determination of increased ventricular mass index (LVMI) by using echocardiography is one of methods to diagnose left ventricular hypertrophy (LVH) in hypertensive patients. The presence of LVH is strongly related with cardiovascular morbidity and mortality (Citation11–14). Carotid intima-media thickness (IMT) can be measured non-invasively by ultrasonographic scan. It is used as a marker for atherosclerotic disease and directly associated with increased risk of cardiovascular events (Citation15,Citation16). Urine albumin–creatinine ratio (UACR) at spot urine sample is used widely and safely to determine presence and severity of proteinuria. Microalbuminuria is a predictor of renal damage and future cardiovascular disease, and associated with presence of LVH and carotid atherosclerosis (Citation17).
In this study, we aimed to investigate the relationship between MPV levels and subclinical TOD in newly diagnosed hypertensive patients.
Material and methods
Study population
Eighty newly diagnosed hypertensive patients (46 male, mean age: 53 ± 5 years) were enrolled to this cross-sectional study between January 2009 to March 2010. An inclusion criterion was presence of newly diagnosed primary essential hypertension, whereas exclusion criteria were evidence of coronary artery disease, chronic heart failure, diabetes mellitus, smoking, renal or hepatic dysfunction, auto-immune disease, hematological disease, cancer, thrombocytopenia, systemic inflammatory conditions and use of any medication. Physical examination and office blood pressure measurements, standard questionnaire to assess history and lifestyle habits, and standard 12-lead ECG were performed to all patients. This study complied with the Declaration of Helsinki, was approved by the Ethics Committee and the institutional review board of Erciyes University Medical School, and informed consent was obtained from each patient.
All of the patients underwent following procedures.
Ambulatory blood pressure monitoring
A portable compact digital recorder was used for 24-h ambulatory blood pressure monitoring (ABPM; Reynolds Medical, Model PG Pressuremeter, Irvine, CA). In the 24-h ABPM, blood pressure measurements were taken at 30-min periods during the night (between 00:00 and 06:00 h) and at 15-min periods during the day (between 06:00 and 24:00 h). An appropriate cuff size was chosen for each subject. The monitor was mounted on the non-dominant arm and removed 24 h later. Hypertension was considered present if the systolic blood pressure (SBP) was >140 mmHg and/or diastolic blood pressure (DBP) was >90 mmHg. According to the ambulatory monitoring, patients with less than 10% decrease in either the SBP or DBP were considered non-dippers in this study.
Echocardiography
Echocardiographic examinations of patients were performed using Vivid 7 system by an experienced cardiologist. The left ventricular mass (LVM) was calculated using the regression equation described by Devereux & Reichek. LVM was divided by surface area, and thus LVMI was calculated. The coefficient of intraobserver variability of LVMI measurements was 1.1 ± 0.2%.
Carotid ultrasonography
All patients were evaluated by high-resolution ultrasound using Vivid 7 system by one experienced physician. Carotid arteries were investigated in the longitudinal plane and lumen diameter was maximized. A region 1 cm proximal to the carotid bifurcation was identified and the carotid IMT of the far wall evaluated as the distance between the lumen– tima interface and the media–adventitia interface. Then, the measurements were taken from both right and left common carotid arteries, the average of both values were calculated. The coefficient of intraobserver variability of carotid IMT measurements was 2.1 ± 0.8%.
Blood sampling
Blood samples were drawn in the morning after a 20-min rest following a fasting period of 12 h. Glucose, creatinine and lipid profiles for blood samples were analyzed for each patient. MPV was measured from tripotassium EDTA-based anticoagulated blood samples drawn in the morning after a 20-min rest, stored at +4°C and assessed by a Sysmex K-1000 autoanalyzer within 30 min of sampling. High-sensitivity C reactive protein (hs-CRP) was measured using a BN2 model nephlometer.
Urine sampling
UACR was measured by using the Beckman Coulter Synchron L × 20 Clinical System.
Statistical analysis
Statistical analysis was done by using SPSS 13.0 statistical software. Adequacy of all parameters to normal distribution was tested by using Kolmogorov–Smirnov Test. Variables that match with normal distribution were given as mean ± SD. Pearson correlation coefficients examined the degree of association between examined variables. Multiple linear regression analyses were performed to identify the significance of the relationship of MPV levels with microalbuminuria, LVMI and carotid IMT. Statistical significance was defined as p < 0.05.
Results
The main clinical characteristics of study population (46 male, mean age was 53 ± 5 years) are presented in . No any patients had disorders such as diabetes mellitus and dislipidemia, and average fasting glucose and lipid levels were in normal range. The mean body mass index was 24.5 ± 3 kg/m2. There were 26 patients with dipper profile while 44 patients showed a non-dipper profile according to ambulatory blood pressure measurements. The average clinical SBP and DBP were 176.5 ± 10 mmHg, 108.5 ± 10.5 mmHg, respectively. Ambulatory measurements revealed that 24-h SBP was 145.8 ± 10.2 mmHg and 24-h DBP was 95.5 ± 5.5 mmHg. The average value of MPV levels was 9.2 ± 0.52 fl, UACR was 0.54 ± 0.05 mg/mmol, LVMI was 111.4 ± 25.8 g/m2, carotid IMT was 0.95 ± 0.20 mm and hs-CRP was 4.2 ± 1.2 mg/lt.
Table I. Clinical characteristics and laboratory findings of study population.
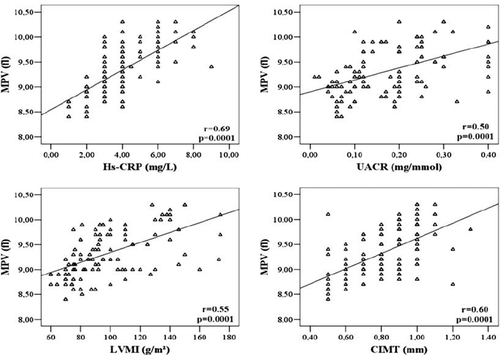
As shown in , MPV is positively correlated with 24-h SBP and DBP (r = 0.51, p = 0.001 and r = 0.55, p = 0.001, respectively). As seen in , correlation analysis showed that MPV was moderately related with severity of proteinuria (r = 0.50, p = 0, 0001), LVMI (r = 0.55, p = 0.0001) and carotid IMT (r = 0.60, p = 0.0001). The other significant correlation was determined between MPV and hs-CRP levels (r = 0.69, p = 0.0001).
Figure 1. Correlation between mean platelet volume (MPV) levels and ambulatory blood pressure measurements. SBP-24 h, 24-h systolic blood pressure; DBP-24 h, 24-h diastolic blood pressure.
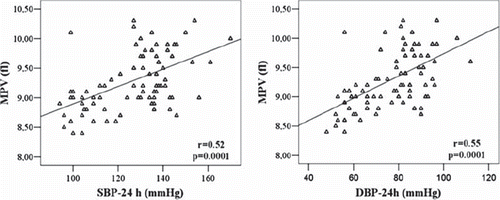
Figure 2. Correlation between mean platelet volume (MPV) levels and high-sensitivity C reactive protein (hs-CRP), urinary albumin–creatinine ratio (UACR), left ventricular mass index (LVMI) and carotid intima media thickness (IMT).
As presented in , multiple linear regression analyses identified that MPV levels were independently associated with severity of proteinuria (coefficient = 0.45, p = 0.001), carotid IMT (coefficient = 0.49, p = 0.001) and LVMI (coefficient = 0.48, p = 0.001). Although most of study patients were overweight, BMI was not determined as an independent factor according to multivariate analysis. hs-CRP level was independently associated with severity of proteinuria (coefficient = 0.42, p = 0.001) and carotid IMT (coefficient = 0.48, p = 0.001) but not with LVMI (coefficient = 0.28, p = 0.08). 24-hSBP and 24-h DBP were other independent factors for severity of proteinuria, carotid IMT and LVMI.
Table II. Multiple linear regression analyses: Relationship between urinary albumin–creatinine ratio (UACR), carotid intima media thickness (IMT), left ventricular mass index (LVMI) and selected variables.
Discussion
Hypertension is a well known risk factor for cardiovascular and cerebrovascular events such as heart attack, renal failure and stroke. In addition, it is associated with earlier changes in target organ systems, such as LVH, proteinuria and carotid atherosclerosis, which are grouped under the term of “target organ damage” (TOD). There are many processes involved in the pathogenesis of TOD and some of these are endothelial dysfunction, platelet activation and increased thrombogenesis (Citation18). MPV is well-established marker of platelet activation. It has been determined that elevated MPV levels were associated with poor prognosis in diseases with increased thrombogenesis including myocardial infarction, ischemic stroke and pulmonary embolism (Citation19–22). Nadar et al. (Citation23) studied 199 hypertensive patients, 125 of them had history of TOD such as stroke, previous myocardial infarction, angina and LVH. They found that MPV was significantly higher in patient with history of clinical TOD compared with patients without history of TOD.
Microalbuminuria is an indicator for a low-level ongoing inflammatory process and it was found that increasing urinary albumin excretion is associated with elevated levels of inflammatory markers, endothelial dysfunction and platelet activation in patients with hypertension (Citation24). Several studies have shown the relationship between platelet activation and microalbuminuria (Citation25,Citation26). In previous studies, it was determined that MPV levels were higher in type 2 diabetic patients who had microvascular complications such as retinopathy or microalbuminuria (Citation27,Citation28). We have found that MPV levels were also correlated with proteinuria levels in hypertensive patients.
Controversial results were obtained about the relationship between carotid IMT and MPV levels from previous studies. Jurcut et al. (Citation29) reported that higher IL-8 level, which is a cytokine with a central role in the inflammatory cascade, is associated with higher MPV and higher carotid IMT. De Luca et al. (Citation30) showed that MPV is not related to the extent of coronary artery disease and carotid IMT but most of patients had taken some sort of medication such as aspirin, clopidogrel, beta-blocker and statin therapy that may affect MPV levels in study group (Citation30). Furthermore, they did not investigate the inflammatory status by using an indicator like hs-CRP. We determined that MPV levels correlated with carotid IMT and hs-CRP levels in newly diagnosed hypertensive patients who did not take any medicine.
Several studies have shown that platelet activation takes an important role in development of LVH (Citation31,Citation32). It is determined that serotonin, platelet-derived growth factor and other factors released from activated platelets increase mass of the myocytes and induce the proliferation of fibroblasts and smooth muscle cells in animal studies (Citation33,Citation34). There is not too much data about relationship between MPV and LVH. Scuteri et al. (Citation35) showed that MPV is associated with increased LVM and interventricular septum thickness in hypertensive patients. In our study, we also demonstrated that MPV levels were associated with levels of LVMI.
hs-CRP is a marker of inflammation and well-established predictor in the detection of patients at increased risk for cardiovascular disease (Citation36). We have determined that hs-CRP level is independently associated with severity of proteinuria and carotid IMT in our study population. Tsioufis et al. (Citation37) showed that increased hs-CRP levels are associated with microalbuminuria, suggesting the involvement of inflammation and endothelial dysfunction in vascular and kidney damage in patients with untreated essential hypertension (Citation37). In a previous study, it was found that CRP is a marker of increased carotid IMT in patients with ischemic stroke (Citation38,Citation39). The other important result of this study is remarkable correlation between MPV and hs-CRP levels. Yazici et al. (Citation40) demonstrated this relationship in rheumatoid arthritis. In our recently published article, we also determined this correlation in non-dipper hypertensive patients (Citation3). This significant correlation determined between MPV and hs-CRP in newly diagnosed hypertensive patients revealed that inflammatory response and platelet activation were beginning at the early onset of hypertension.
We demonstrated that MPV levels were correlated with 24-h ambulatory blood pressure measurements. Several previous studies also showed this relationship. In our recently published study, we found that MPV levels were higher in hypertensive patients and especially in non-dippers (Citation10). Coban et al. (Citation41) found that MPV was positive correlated with ambulatory DBP in essential hypertension and white coat hypertension groups. Our results were in agreement with these previous studies. Furthermore, we determined that 24-h SBP and 24-h DBP are independently associated with severity of proteinuria, carotid IMT and LVMI. Several previous studies have shown this association. Manios et al. (Citation42) showed that 24-h SBP variation is independently associated with impaired renal function in hypertensive patients. Ekart et al. (Citation43) found statistically significant correlation between carotid IMT and DBP on the ABPM measurements in hemodialysis patients. Moreover, Ozawa et al. (Citation44) found that LVH was independently associated with 24-h SBP and 24-h DBP measurements (Citation44).
One of the limitations of this study may be method of MPV measurement. Tripotassium EDTA-based anticoagulated blood samples were used to measure MPV levels in our study. Most laboratories use EDTA for anticoagulation of whole blood prior to automated cell counting but because of platelet swelling, MPV values may increase with its use (Citation45). Dastjerdi et al. (Citation46) found that MPV can be measured accurately by using anticoagulation, EDTA and citrate if the analysis is performed within 1 h of sampling. Macey et al. (Citation47) also showed the changes in MPV, which reflect platelet sphering and swelling, were greatest after between 30 and 60 min in blood stored at ambient temperature, whereas in our study, blood samples were stored at ±4°C and measurement performed within 30 min. So we were adequately confidence about results in our study.
In conclusion, our findings suggested that MPV levels were associated with severity of subclinical TOD including carotid atherosclerosis, LVH and renal damage in hypertensive patients. In addition to this, MPV levels were significantly correlated with hs-CRP levels and 24-h ambulatory blood pressure measurements.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lande K, Os I, Kjeldsen SE, Westheim A, Hjermann I, Eide I, . Increased platelet size and release reaction in essential hypertension. J Hypertens. 1987;5:401–406.
- Lande K, Kjeldsen SE, Os I, Westheim A, Hjermann I, Eide I, . Increased platelet and vascular smooth muscle reactivity to low-dose adrenaline infusion in mild essential hypertension. J Hypertens. 1988;6:219–225.
- Kaya MG, Yarlioglues M, Gunebakmaz O, Gunturk E, Inanc T, Dogan A, . Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209:278–282.
- Nadar S, Lip GY. The prothrombotic state in hypertension and the effects of antihypertensive treatment. Curr Pharm Des. 2003;9:1715–1732.
- Islim IF, Bareford D, Ebanks M, Beevers DG. The role of platelets in essential hypertension. Blood Press. 1995;4:199–214. Review.
- Lip GY. Target organ damage and the prothrombotic state in hypertension. Hypertension. 2000;36:975–977.
- Kamath S, Blann AD, Lip GY. Platelet activation: Assessment and quantification. Eur Heart J. 2001;22:1561–1571.
- Bath PM, Butterworth RJ. Platelet size: Measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996; 7:157–161.
- Park Y, Schoene N, Haris W. Mean platelet volume as an indicator of platelet activation: Methodological issues. Platelets. 2002;13:301–306.
- Inanc T, Kaya MG, Yarlioglues M, Ardic I, Ozdogru I, Dogan A, . The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010;19:81–85.
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implication of echocardiographic determined left ventricular mass in the Framingham Heart Study. N Eng J Med. 1990;322:1561–1556.
- Vakili BA, Okin PM, Devereux RB. Prognostic significance of left ventricular hypertrophy. Am Heart J. 2001;141: 334–341.
- De Simone G, Verdecchia P, Pede S, Gorini M, Maggioni AP. Prognosis of inappropriate left ventricular mass in hypertension The MAVI study. Hypertension. 2002;40:470–476.
- Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, . Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk In Communities (ARIC) study. Am J Epidemiol. 1997;146:483–494.
- Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimamedial thickness is related to cardiovascular risk factors measured from childhood through middle age. Circulation. 2001; 104:2814–2819.
- Leoncini G, Sacchi G, Ravera M, Viazzi F, Ratto E, Vettoretti S, . Microalbuminuria is an integrated marker of subclinic organ damage in primary hypertension. J Hum Hypertens. 2002;16:399–404.
- Nadar SK, Tayebjee MH, Messerli F, Lip GY. Target organ damage in hypertension: Pathophysiology and implications for drug therapy. Curr Pharm Des. 2006;12:1581–1592.
- Pizzulli L, Yang A, Martin JF, Lüderitz B. Changes in platelet size and count in unstable angina pectoris compared to stable or non-cardiac chest pain. Eur Heart J. 1998;19:80–84.
- Kostrubiec M, Labyk A, Pedowska-Wloszek J, Hrynkiewicz Szymanska A, Jankowski K, Pacho S, . Mean platelet volume predicts early death in acute pulmonary embolism. Heart. 2009 Nov 11. [Epub ahead of print]
- Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of CVD. Stroke. 2004;35:622–626.
- Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, . Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J Thromb Haemost 2010;8:148–156.
- Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY Platelet indexes in relation to target organ damage in high-risk hypertensive patients: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol. 2004; 44:415–422.
- Kalaitzidis R, Bakris G. Pathogenesis and treatment of microalbuminuria in patients with diabetes: The road ahead. J Clin Hypertens. 2009;11:636–643.
- Guagnano MT, Ferroni P, Santilli F, Paoletti V, Manigrasso MR, Pescara L, . Determinants of platelet activation in hypertensives with microalbuminuria. Free Radic Biol Med. 2009;46:922–927.
- Ferroni P, Guagnano MT, Falco A, Paoletti V, Manigrasso MR, Michetti N, . Association of low-grade inflammation and platelet activation in patients with hypertension with microalbuminuria. Clin Sci. 2008;114:449–455.
- Hekimsoy Z, Payzin B, Ornek T, Kando an G. Mean platelet volume in Type 2 diabetic patients. J Diabetes Complications. 2004;18:173–176.
- Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, . Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–478.
- Jurcut R, Arsenescu I, Pu cariu T, Usc tescu V, Jurcu C, Apetrei E, . Is interleukin-18 correlated with endothelial dysfunction and platelet activation in patients with unstable angina? Rom J Intern Med. 2005;43:199–209.
- De Luca G, Santagostino M, Secco GG, Cassetti E, Giuliani L, Franchi E, . Mean platelet volume and the extent of coronary artery disease: Results from a large prospective study. Atherosclerosis. 2009;206:292–297.
- Gabbasov Z, Parfyonova Y, Popov E, Gavrilov I, Anuchin V, Dubov P, . Associations of platelet function in hypertensive patients with left ventricular hypertrophy, transient myocardial ischemia, and coronary artery disease. Platelets. 1998;9:191–195.
- Lip GY, Blann AD. Associations of platelet function in hypertensive patients with left ventricular hypertrophy. Platelets. 1999;10:71–72.
- Yabanoglu S, Akkiki M, Seguelas MH, Mialet-Perez J, Parini A, Pizzinat N. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol. 2009;46:518–525.
- Wang C, Wu LL, Liu J, Zhang ZG, Fan D, Li L. Crosstalk between angiotensin II and platelet derived growth factor-BB mediated signal pathways in cardiomyocytes. Chin Med J. 2008;121:236–240.
- Scuteri A, Cacciafesta M, De Propris AM, Di Bernardo MG, Recchi D, Celli V, . Platelet size and left ventricular hypertrophy in hypertensive patients over 50 years of age. Eur J Clin Invest. 1995;25:874–876.
- Rifai N, Ridker PM. High-sensitivity C-reactive protein: A novel and promising marker of coronary heart disease. Clin Chem. 2001;47:403–411.
- Tsioufis C, Dimitriadis K, Andrikou E, Thomopoulos C, Tsiachris D, Stefanadi E, . ADMA, C-reactive protein, and albuminuria in untreated essential hypertension: A cross-sectional study. Am J Kidney Dis 2010 Feb 25 [Epub ahead of print].
- Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, . Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: The Cardiovascular Health Study. Circulation. 2007;116:32–38.
- Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, . C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: The Cardiovascular Health Study. Circulation. 2003;108:166–170.
- Yazici S, Yazici M, Erer B, Erer B, Calik Y, Ozhan H, Ataoglu S. The platelet indices in patients with rheumatoid arthritis: Mean platelet volume reflects disease activity. Platelets. 2010; 21:122–125.
- Coban E, Yazicioglu G, Berkant Avci A, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets. 2005;16:435–438.
- Manios E, Tsagalis G, Tsivgoulis G, Barlas G, Koroboki E, Michas F, . Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens. 2009 Jul 30 [Epub ahead of print].
- Ekart R, Hojs R, Pecovnik-Balon B, Bevc S, Dvorsak B. Blood pressure measurements and carotid intima media thickness in hemodialysis patients. Ther Apher Dial. 2009;13: 288–293.
- Ozawa M, Tamura K, Okano Y, Matsushita K, Ikeya Y, Masuda S, . Blood pressure variability as well as blood pressure level is important for left ventricular hypertrophy and brachial–ankle pulse wave velocity in hypertensives. Clin Exp Hypertens. 2009;31:669–679.
- Kjeldsen SE, Weder AB, Egan B, Neubig R, Zweifler AJ, Julius S. Effect of circulating epinephrine on platelet function and hematocrit. Hypertension. 1995;25:1096–105.
- Dastjerdi MS, Emami T, Najafian A, Amini M. Mean platelet volume measurement, EDTA or citrate? Hematology. 2006; 11:317–319.
- Macey M, Azam U, McCarthy D, Webb L, Chapman ES, Okrongly D, . Evaluation of the anticoagulants EDTA and citrate, theophylline, adenosine, and dipyridamole (CTAD) for assessing platelet activation on the ADVIA 120 hematology systems. Clin Chem. 2002;48:891–899.