Abstract
Background. Arterial hypertension is commonly associated with hyperuricemia. Several studies have shown that allopurinol reduces arterial blood pressure in animal models and in adolescent patients with newly diagnosed hypertension. Moreover, allopurinol has shown beneficial effects on endothelial function and arterial wave reflection in contrast to uricosuric agents. Antihypertensive drugs produce different effects on serum uric acid levels. Objective. The aim of the study was to evaluate the influence of allopurinol on blood pressure and aortic compliance in patients with arterial hypertension depending on hypotensive therapy with angiotensin-converting enzyme inhibitor (ACE-I) or thiazide diuretic, hypotensive drugs with distinct effects on serum uric acid levels and conversely, a positive influence on pulse wave velocity (PWV) in the aorta. Material and Methods. Sixty-six patients aged 25–70 (mean age 46.17 ± 10.89) with mild and moderate arterial hypertension diagnosed on the basis of office blood pressure, were studied. They were randomized to antihypertensive therapy on either perindopril (n = 35) or hydrochlorothiazide (n = 31). After 8 weeks of antihypertensive therapy, 150 mg of allopurinol daily was added for the next 8 weeks. Measurement of the serum uric acid level, PWV and 24-h ambulatory blood pressure monitoring (ABPM) were performed at baseline, after 8 weeks antihypertensive therapy and again after the final 8 weeks with the additional allopurinol. Results. No significant changes in systolic (SBP) and diastolic blood pressure (DBP) or ABPM were observed after allopurinol treatment in either of the subgroups receiving ACE-I or thiazide-based antihypertensive therapy. The mean PWV decreased from 10.7 ± 1.4 m/s to 10.0 ± 1.2 m/s (p = 0.00008) in the ACE-I-based therapy subgroup and from 11.5 ±1.7 m/s to 10.4 ± 1.5 m/s (p = 0.00002) in the thiazide-based therapy subgroup after treatment with allopurinol. However, significant correlations were found between PWV changes and the basic PWV (r = −0.52; p < 0.001) or SBP changes (r = 0.29; p < 0.019) after allopurinol treatment. Conclusions. Allopurinol does not produce additional antihypertensive effects in patients with treated arterial hypertension. Allopurinol increases aortic compliance independently of ACE-I or thiazide-based, antihypertensive therapy. However, this effect is significantly dependent on the initial PWV in the aorta and on SBP changes during allopurinol therapy.
Introduction
There is growing interest in the relationship between serum uric acid and cardiovascular diseases. Several recent studies indicate that hyperuricemia might be an independent predictor of cardiovascular complications in patients with arterial hypertension (Citation1–7).
However, there is very little data about the influence of allopurinol on blood pressure levels. In rats with hyperuricemia, allopurinol decreased levels of serum uric acid and reduced blood pressure (Citation8). In another study, involving dexamethasone-induced hypertensive rats, allopurinol reduced both serum uric acid levels and blood pressure (Citation9). However, previous studies had not revealed such a positive effect of this xanthine oxidase inhibitor on blood pressure (Citation10). Some of the studies concerning the influence of allopurinol on blood pressure were conducted in men. In a small, pilot study, normalization of blood pressure was observed in four out of five men after allopurinol therapy (Citation11). In patients with type 2 diabetes mellitus and mild arterial hypertension (Citation12) and also in patients with chronic heart failure (Citation13), allopurinol did not have a hypotensive effect. The study of Feig et al. (Citation14), regarding young people with newly diagnosed untreated hypertension, revealed that allopurinol significantly reduced blood pressure measured in the office and during ambulatory blood pressure monitoring (ABPM).
A possible mechanism of allopurinol's hypotensive effect may be connected to the beneficial influence of inhibition of xanthine oxidase on endothelial function, which was observed in patients with hypertension, diabetes mellitus, coronary heart disease and heart failure (Citation12,Citation15).
The influence of allopurinol on aortic compliance, an important factor affecting blood pressure, has not yet been assessed in hypertensive patients. Therefore, in view of the divergent results reported in the literature, the aim of the current study was to evaluate the influence of allopurinol on blood pressure and aortic compliance in patients with arterial hypertension treated with angiotensin-converting enzyme inhibitors (ACE-I) or thiazide diuretics, hypotensive drugs with differential effects on serum uric acid levels.
Methods
Subjects
We have recruited 84 patients with I and II stage arterial hypertension (according to ESH/ESC 2007 criteria) from the outpatient clinics of family physicians in Poznan. The study was performed in the Department of Hypertension, Angiology and Internal Medicine of Poznan University of Medical Sciences.
The inclusion criteria were written consent, an age between 25 and 70 years old, and untreated mild or moderate arterial hypertension. Patients with secondary arterial hypertension, “white coat hypertension”, and concomitant diseases (diabetes mellitus, cardiomiopathy, valve defects, systolic heart failure, hematological diseases, malignant neoplasms, hepatic cirrhosis, kidney failure, neurological and mental diseases) were excluded from the study. Additional exclusion criteria were women in gestational age planning pregnancy, patients with atrial fibrillation or other arrythmias, and those with a number of biochemical abnormalities such as glucose >7.0 mmol/l, potassium <3.5 mmol/l or >5.5 mmol/l and creatinine >1.2 mg/dl.
All the patients gave their written consent after receiving exhaustive information about the study (in oral and written form). The details of the study were described in the study protocol, which was approved by the Bioethics Committee of Poznan University of Medical Science.
Study design
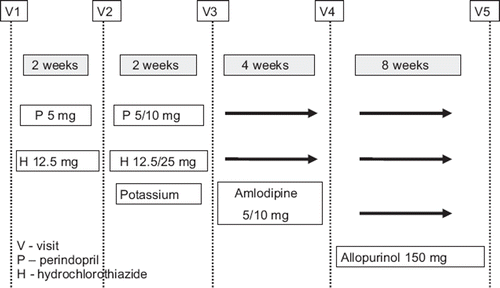
Patients enrolled in the study made five visits over a period of 4 months according to the study protocol (). During the first visit (V1), a full medical history was taken and physical examination. Measurement of the blood pressure in the sitting position was performed three times. Patients were qualified to the study on the basis of the blood pressure measurement. Analysis of basic laboratory parameters, including the serum uric acid level, was carried out. Pulse wave velocity (PWV) measurement was performed and an ABPM device was put on. The ABPM results were noted the following day and random treatment with ACE-I (perindopril) or thiazide (hydrochlorothiazide) was initiated.
The second visit (V2) was after 2 weeks of active treatment. Assessment of blood pressure was performed and, if necessary, the dose of hypotensive drug was doubled; in the case of perindopril to 10 mg and of hydrochlorothiazide to 25 mg. After a further 2 weeks of active treatment, the third visit (V3) was made. In cases of unsatisfactory blood pressure control (when BP > 140/90 mmHg), a second hypotensive drug, the calcium-channel blocker, amlodipine, was added in a dose of 5/10 mg).
The fourth visit (V4) took place after next 4 weeks of active hypotensive treatment. The examinations carried out at the first visit were repeated and, additionally, allopurinol in a dose of 150 mg was administered.
The last visit (V5) was made after 2 months of active treatment with allopurinol and included the same examinations as those made during visits V1 and V4.
Measurements
The blood pressure was measured with an OMRON-705IT electronic manometer. This model has been approved by the European Society of Hypertension, having fulfilled the evaluation criteria of the Association for the Advancement of Medical Instrumentation and the British Hypertension Society.
Twenty-four-hour ABPM was carried out on a 2430TM device (A&D company). This model also has been approved by the European Society of Hypertension, having fulfilled the evaluation criteria of the Association for the Advancement of Medical Instrumentation and the British Hypertension Society.
The blood pressure was measured between 08:00 and 10.00 h. Examinations were performed on working days only. The frequency of measurement was every 15 min during the day and 30 min at night. In the final statistical analysis, only readings with more than 90% of valid measurements were included.
Aortic compliance was assessed with a non-invasive method of measuring the PWV, on the COMPLIOR® computer system. The examination was performed in the morning after 5 min rest in a lying position. Pressurized converters TY-306 (Fukuda Co., Japonia), placed where the carotid and the femoral artery was the most palpable, were used. The measurement had a signal frequency sampling of 0.03–300 Hz. The computer program performed an evaluation of the PWV dividing the distance between converters by the time that the pulse wave needed to move from the carotid to the femoral artery. The time was calculated as the lag between the beginning of the pulse curve from the carotid and the femoral artery and the distance between the converters. The distances between the pulse wave registration points on the carotid and the femoral arteries was measured. The final value of the PWV was the mean of 20 measurements, where the two extreme results were rejected.
The serum uric acid level was assessed by mean of a Dimension χ Pand Plus analyzer (Dade Behring), with the use of a Flex reagent refill.
Statistical analysis was performed using the Statistica PL v. 8.0 program (Statsoft Polska.5).
Results
Sixty-six patients (40 men, 26 women) completed the study. The mean age of patients was 46.2 ± 10.9 years. Baseline hyperuricemia was noted in 16.8% of the patients. It was present more frequently in men (eight patients, 20%) than in women (three patients, 11.5%). Thirty-five patients were treated with perindopril (group P) and 31 patients were treated with hydrochlorothiazide (group H). The baseline characteristics of both groups are presented in .
Table I. Baseline characteristics of the perindopril (P) and hydrochlorothiazide (H) groups.
Groups P and H did not differ significantly with respect to age and gender. The clinical and biochemical parameters in both groups were similar and initial SBP, DBP, PP in office and ABPM measurements (V1) in both groups did not differ significantly ( and ).
Table II. Office blood pressure in the perindopril (P) and hydro-chlorothiazide (H) groups.
Table III. Ambulatory blood pressure (ABPM) in the perindopril (P) and hydrochlorothiazide (H) groups.
The mean SBP and DPB significantly decreased after 8 weeks of hypotensive therapy in group P and group H. The reduction in SBP and DBP in both groups was similar (23.5 vs 23.8 mmHg; p = 0.92 and 13.8 vs 12.2 mmHg; p = 0.48, respectively).
We did not observe significant differences in office and ABPM blood pressure reduction between group P and H ( and ).
PWV decreased significantly from 11.6 ± 1.7 to 10.7 ± 1.4 m/s (p < 0001) after 8 weeks of hypotensive therapy in group P, whereas it did not change (11.9 ± 1.7 vs 11.6 ± 1.7 m/s) in group H.
The serum uric acid level decreased significantly during allopurinol therapy in group P from 5.46 ± 1.17 mg/dl to 4.96 ± 1.34 mg/dl (p < 0.001), and in group H from 5.75 ± 1.63 to 4.84 ± 1.21 mg/dl (p < 0.001). The hypouricemic effect of allopurinol was similar in both groups (0.77 vs 0.90 mg/dl; p = 0.46).
Effect of allopurinol on office BP
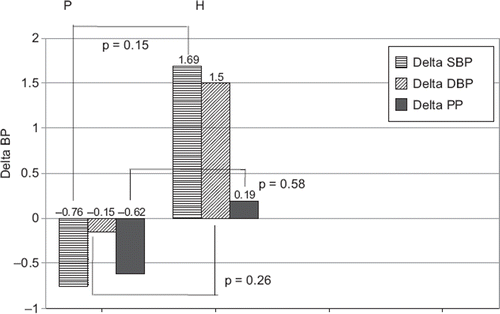
There was a tendency to a reduction in BP during therapy with allopurinol in group P and a tendency to increase in group H. However, these differences were not significant ( and ). In both groups, a significant correlation between the initial SBP after hypotensive therapy and a change in SBP during further treatment with allopurinol was revealed (r = −0.377, p = 0.026 and r = −0.441, p = 0.013, respectively).
Effect of allopurinol on BP in ABPM
Also, in ABPM, the tendency to a reduction in blood pressure during therapy with allopurinol in group P and tendency to increase in group H were observed. However, the differences between groups were not significant ().
Effect of allopurinol on PWV
After 8 weeks of therapy with allopurinol, PWV decreased from 11.1 ± 1.6 to 10.2 ± 1.4 m/s (Δ −0.91) for the whole investigated group ().
Figure 3. Influence of allopurinol therapy on pulse wave velocity (PWV) in groups perindopril + hydrochlorothiazide (P + H), P and H.
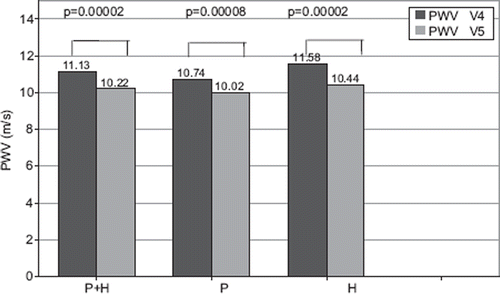
The mean PWV in group P during V4 was 10.7 ± 1.5 m/s, and during V5 10.0 ± 1.2 m/s (p = 0.00008). In group H, it was 11.6 ± 1.7 m/s and 10.4 ± 1.6 m/s, respectively (p = 0.00002). The effect of allopurinol on PWV tended (p = 0.08) to be stronger in group H (−1.12 m/s) than in group P (−0.72 m/s) ().
Figure 4. Correlation between pulse wave velocity (PWV) baseline after antihypertensive therapy (perindopril + hydrochlorothiazide, P + H) and between the change of PWV while receiving allopurinol.
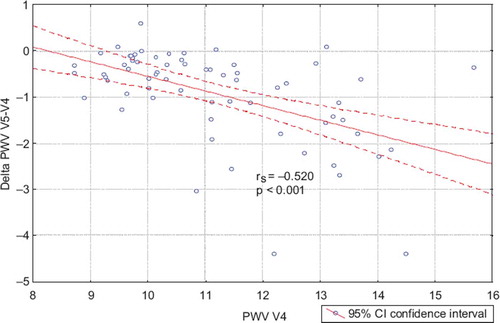
A significant correlation was also found between basic PWV and PWV changes after allopurinol treatment (r = −0.520; p < 0.001) (). This relationship was also significant in both subgroups P and H.
Discussion
The novel findings of this study are, first, that allopurinol does not have an accessory hypotensive effect in patients with arterial hypertension treated with either ACE-I or thiazide-based antihypertensive therapy. Second, allopurinol increases aortic compliance irrespective of the antihypertensive drugs used. However, this effect is significantly dependent upon initial PWV in the aorta and changes in systolic and diastolic blood pressure during antihypertensive therapy.
The results of previous human studies assessing the influence of allopurinol on blood pressure are not consistent. In a small, pilot study, during 1-month therapy with allopurinol in a dose of 400 mg/day, normalization of blood pressure was observed in four out of five men (Citation11). Kanbay et al. (Citation16) reported a significant blood pressure decrease after 12 weeks of therapy with allopurinol in a daily dose of 300 mg. In a small study of 28 post-stroke patients, allopurinol in a dose of 300 mg/day decreased SBP and DBP insignificantly (Citation17). Allopurinol did not have a hypotensive effect in patients with type 2 diabetes mellitus and mild arterial hypertension (Citation12) as well as in patients with chronic heart failure (Citation13). Some of papers suggest that allopurinol has no beneficial effect in healthy subjects (Citation18). However, Feig et al. (Citation14) have shown that allopurinol decreases both office and ABPM blood pressure in young patients with hyperuricemia and primary arterial hypertension. In their study, hypotensive effect of allopurinol was comparable with a typical hypotensive agent. This study had a relatively short-term duration (1 month) and the effect of education about diet and physical activity is hard to evaluate. Another reason for distinct results may be a different dose of allopurinol. In our study, the dose of allopurinol was 150 mg/day and in Feig et al. (Citation14) it was 400 mg/day. It is noticeable that in the study of Feig et al. (Citation14), a young population with arterial hypertension and hyperuricemia, but without organ damage, was enrolled and allopurinol was the only administered drug. Moreover, according to the literature, hyperuricemia is frequent in young people with arterial hypertension, i.e. around 90% of cases (Citation18), but in an adult population with arterial hypertension, according to our previous experience, it is around 15% (Citation19). We enrolled adult patients, not treated before, with different types of organ damage from our study, and allopurinol was added after 2 months of active hypotensive treatment, so in patients with normal or near normal blood pressure. We have to remember that with age, and also with duration of arterial hypertension, a number of structural changes take place in the aorta such as degeneration and destruction of elastic fibers, with its gradual replacement by collagen ones, thereby reducing elasticity. The number of polyploid muscular cells, which contain fewer contractile elements and more inextensible nuclear material, is increased. Moreover, with increasing age, calcium deposits accumulate in the aorta because of atherosclerosis. As a result of these changes, the aorta wall becomes stiff and less compliant. Impaired aortic compliance in adult patients causes distinct hemodynamic changes from those in the young arteries.
Our results suggest an improvement of the aortic compliance with allopurinol. A dose of 150 mg daily caused a significant reduction in the PWV. The influence of allopurinol on this parameter was slightly stronger in the group treated with hydrochlorothiazide. In both groups, the the PWV reduction was greater when the value of this parameter was higher at V4 during therapy. One cannot rule out that the decrease in PWV between V4 and V5 was partly caused by prolonged antihypertensive treatment; however the findings that PWV did not change significantly between V1 and V4 in group H and that the decrease in PWV was higher in group H after allopurinol treatment, despite beneficial effect of ACE inhibitor but not thiazide on PWV, makes this option less possible. However, the lack of an arm with prolonged antihypertensive treatment without allopurinol treatment is a limitation of our study.
The mechanism by which allopurinol improves the PWV in the aorta is not clear. The tendency for better improvement of aortic compliance in cases of considerable reduction of serum uric acid suggests a direct role in hypouricemia. An indirect mechanism of improvement of endothelial function by allopurinol is also likely. We have to remember that this improvement is connected with reduced production of free radicals during the transformation of xanthine and hypoxanthine to uremic acid by xanthine dyhydrogenase under hypoxemic condition.
The effect of allopurinol on aortic compliance has been studied in patients with chronic kidney failure (Citation20). In this study hbPWV (heart brachial PWV) decreased significantly in patients receiving allopurinol in a dose 100 mg daily. It is recommended, however, that PWV should be measured between the carotid and the femoral artery. Indirect confirmations of the beneficial effect of allopurinol on aortic compliance are the results of a study that investigated the influence of allopurinol on the central blood pressure in patients after stroke (Citation17). The 28 patients with hyperuricemia received 300 mg of allopurinol daily for 8 weeks on top of standard treatment. At the beginning of the study and after 8 weeks, traditional blood pressure measurement, central pressure wave analysis and arterial wave reflection were determined from the augmentation index (AIx), which was adjusted for heart rate 75 (AIx75) and time of return of reflected wave (Tr). Aix results from the presence of a wave reflected from resistance vessels, and depends on many factors potentially modifiable by medications. In their group receiving allopurinol, AIx significantly declined, whereas it significantly increased in the placebo group. No significant correlations between changes of AIx75 and changes of serum uric acid were observed. The time of the reflected wave's return was slightly increased after therapy with allopurinol, which informs about the decrease of pulse wave. Nevertheless no significant changes of SBP and DBP in traditional measurements were observed in this study (Citation17). A decrease in PWV did not correlate with a hypouricemic effect in our study. Similar results were observed by Baldus et al. (Citation21), who showed that inhibition of xanthine oxidase by oxypurinol improved endothelial function in patients with ischemic heart disease independently of serum uric acid changes. Such an observation suggests that allopurinol might have a beneficial effect on aortic compliance through a path other than a hypouricemic one. However, Mercuro et al. (Citation22) revealed that in patients with a higher cardiovascular risk, improvement of endothelial function depends on uricemia. One of the mechanisms of allopurinol's action on a vessels’ compliance is inhibition of the super oxide form of radical formation and a reduction of oxidative stress in vessel walls (Citation23). This latter research confirmed the relationship between AIx, pulse pressure and super oxide anion production. In another study, on patients with hyperuricemia and with normal renal functions, treatment with 300 mg of allopurinol for 3 months caused a decrease of C-reactive protein (CRP) and blood pressure (Citation16). An elevated level of CRP is connected with an increased augmentation index (Citation24), so it is possible that allopurinol decreases AIx through an anti-inflammatory process. A relationship between increased artery stiffness and the inflammatory process in primary arterial hypertension was detected recently. Associations were reported between artery stiffness, tumor necrosis factor (TNF-α), interleukin 6 (IL-6) and CRP as assessed by a highly specific method (hs-CRP) (Citation25).
The study of George et al. (Citation26) revealed the beneficial effects of allopurinol on endothelium function independently of hypouricemic effect. The authors compared the results of 1 month's therapy with allopurinol, probenecid vs a placebo. Allopurinol caused a significant increase of blood flow after acetylcholine infusion. Increasing the daily dose of allopurinol from 300 to 600 mg additionally improved endothelial function, while additional reduction of uricemia was relatively small. Probenecid, which is an uricosuric drug, decreased serum uric acid comparably with allopurinol but had no effect on endothelial function. A simultaneous infusion of acetylcholine and vitamin C revealed that 600 mg of allopurinol completely reduces oxidative stress susceptible to high doses of vitamin C. Although this study did not assess the influence of allopurinol on PWV, it did give some important data supporting our results. The beneficial effect of allopurinol on endothelial function was dose-dependent and was not directly related to hypouricemic effect, since probenecid had no effect on blood flow, and lastly, allopurinol was a stronge antioxidant. Several other studies confirmed the beneficial effect of allopurinol on endothelial function (Citation13–15).
In summary, the most important observation from our study is a positive effect of xanthine oxidase inhibitor on aortic compliance. Such an effect is possible, in spite of the absence of a hypotensive action, if we take into consideration the beneficial effect on endothelial function previously reported. Improvement of arterial function by allopurinol is revealed even if ACE-I, which itself has an independent, beneficial effect on aortic compliance, is used. We cannot exclude the possibility that a hypotensive effect of allopurinol is present, but conclude that during hypotensive treatment, it is not large enough to be detected, despite an improvement in aortic compliance. This hypothesis was suggested by the inverse relationships between initial blood pressure during hypotensive treatment with ACE-I or thiazide diuretic, before treatment with allopurinol, and changes of blood pressure after adding allopurinol. The results presented here do not permit the unequivocal conclusions to be drawn that allopurinol should be used in all patients at higher cardiovascular risk. However, the results concerning the influence of this drug on aortic compliance prompt the necessity for clinical trials in order to confirm this hypothesis.
Potential conflict of interest: None.
References
- Langford HG, Blaufox MD. Is thiazide-produced uric acid elevation harmful? Analysis of data from the Hypertension Detection Follow-up Program. Arch Intern Med. 1987;147: 645–650.
- Franse LV. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program. J Hypertens. 2000;18:1149–1154.
- The Hypertension Detection and Follow-up Program Cooperative Research Group. Mortality findings for stepped-care and referred-care participants in the hypertension detection and follow-up program, stratified by other risk factors. Prev Med. 1985;14:312–335.
- Staessen J. The determinants and prognostic significance of serum uric acid in elderly patients of the European Working Party on High Blood Pressure in the Elderly trial. Am J Med. 1991;90:50–54.
- Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150.
- Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–1078.
- Wang JG, Staessen JA, Fagard RH, Birkenhager WH, Gong L, Liu L, for the Systolic Hypertension in China (Syst-China) Trial Collaborative Group. Prognostic significance of serum creatinine and uric acid in older Chinese patients with isolated systolic hypertension. Hypertension. 2001;37: 1069–1074.
- Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, . Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1110.
- Wallwork CJ, Parks DA, Schmid-Schonbein GW. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003;66:30–37.
- Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hypertensive rats. J Hypertens. 2004;22:1333–1340.
- Feig DI, Nakagawa T, Karumanchii SA, Oliver WJ, Kang D-H, Finch J, . Nephron number, uric acid, and renal microvascular disease in the pathogenesis of essential hypertension. Kidney Int. 2004;66:281–287.
- Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751.
- Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, . Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation. 2002;105: 2619–2624.
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. A randomized trial. JAMA. 2008;300:924–932.
- Farquharson CAJ, Butler R, Hill A, Belch JJF, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226.
- Kanbay M, Ozkara A, Selcky Y, Isik B, Turgut F. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233.
- Khan F, George J, Wong K, McSwiggan S, Struthers AD, Belch JJ. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc Therapeutics. 2008;26:247–252.
- Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2007;49:247–252.
- Kostka-Jeziorny K, Tykarski A. Związek hiperurykemii z innymi czynnikami ryzyka sercowo-naczyniowego u pacjentów z pierwotnym, nieleczonym nadciśnieniem tętniczym w populacji badania RISK. Nadciśnienie Tętnicze. 2008;3:190–199 (in Polish).
- SriBhushan Raju D, Ram Mohan P, Naidu MU. Effect of allopurinol on arterial stiffness and endothelial function in patients with chronic renal failure. Ind J Nephrology. 2007; 17:4–8.
- Baldus S, Koster R, Chumley P, Heitzer T, Rudolph V, Ostad MA, . Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190.
- Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, . Effect of hyperuricemia upon endothelial function in patients with increased cardiovascular risk. Am J Cardiol. 2004;94:932–935.
- Wykretowicz A, Guzik P, Kasinowski R, Krauze T, Bartkowiak G, Dziarmaga M, . Augmentation index, pulse pressure amplification and superoxide anion production in patients with coronary artery disease. Int J Cardiol. 2005;99:289–294.
- Kullo IJ, Seward JB, Bailey KR, Bielak LF, Grossardt BR, Sheedy PF, . C-reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens. 2005;18:1123–1129.
- Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46; 1118–1122.
- George J, Carr E, Davies J, Belch JJF, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516.