Abstract
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase and is associated with endothelial dysfunction. The aim of the present study was to investigate the relationship between ADMA, indices of arterial stiffness, endothelial function and carotid artery intima-media thickness (IMT) in hypertension patients. Eighty middle-aged (47 ± 10 years) untreated patients with mild to moderate essential hypertension underwent routine physical examination, pulse wave analysis (PWA), measurement of aortic pulse wave velocity (PWV) and IMT. In PWA, administration of salbutamol and nitroglycerine was used to assess endothelium-dependent (EDV) and endothelium-independent vasodilation, respectively. In univariate analysis, ADMA was correlated with EDV (r = −0.26; p = 0.02) and IMT (r = 0.32; p = 0.007). In multiple regression analysis, ADMA was independently associated with the female gender, EDV, IMT and total cholesterol (R2 = 0.30; p < 0.001). No correlation was detected between ADMA and augmentation index, central/brachial blood pressure or aortic PWV. In hypertension patients, ADMA is independently correlated with IMT and EDV. Thus, ADMA is a marker of endothelial dysfunction and intima-media thickening in patients with hypertension.
Introduction
Hypertension is the leading cause of cardiovascular death globally and affects approximately 1 billion people worldwide (Citation1). Despite the several traditional risk factors known in hypertension, they do not fully explain cardiovascular risk in these patients. Structural and functional integrity of the endothelium is essential for prevention of atherosclerosis in the arteries. Nitric oxide (NO), which is produced in the endothelium from l-arginine by endothelial nitric oxide synthase (e-NOS), is the main vasodilating and a major antiatherogenic biomolecule in the human arteries (Citation2). NO-mediated endothelium-dependent vasodilation (EDV), the hallmark of endothelial function, is decreased in hypertension (Citation3). Asymmetric dimethylarginine (ADMA), an inhibitor of e-NOS, has been shown to be increased in hypertension and may be causally involved in endothelial dysfunction (Citation4,Citation5). It has been shown previously that pulse wave analysis (PWA) with pharmacological tests is a non-invasive, reproducible and simple method for assessment of global arterial endothelial function (Citation6). Intima-media thickness of the carotid arteries (IMT) reflects early structural atherosclerotic changes in the arteries (Citation7). The aim of this study was to evaluate correlations between vascular function indices measured by PWA, IMT and ADMA in patients with essential hypertension.
Materials and Methods
Subjects
Eighty subjects (41 men, 39 women) with untreated mild to moderate essential hypertension, aged 47 ± 10 years, were recruited into the study from a hypertension outpatient clinic or from local general practices. All subjects underwent a physical examination and a review of their medical history. Mild or moderate hypertension was defined as systolic blood pressure 140–179 mmHg and/or diastolic blood pressure 90–109 mmHg on at least two occasions with a 1-month interval. Subjects with diabetes mellitus (fasting plasma glucose > 6.4 mmol/l), ischaemic heart disease, clinically relevant heart failure (NYHA class II–IV), renal failure, bronchial asthma, malignancies, body mass index (BMI) > 30 kg/m2, acute or chronic inflammatory disease, hypercholesterolaemia (total cholesterol > 6.5 mmol/l) and secondary hypertension and smokers (>10 cigarettes a day) were excluded. The study protocol was approved by the Ethics Committee, University of Tartu. Informed written consent was obtained from each participant in accordance with the principles of the Declaration of Helsinki.
Study protocol
All studies were conducted at the Endothelial Centre, University of Tartu, Estonia. The patients were studied and plasma samples were obtained between 08:00 and 11:00 h after an overnight fast and abstinence from any medication, tobacco, alcohol, tea and coffee. After sitting for at least 15 min in a quiet, temperature-controlled room, blood pressure was measured and after a brief period of rest, aortic pulse wave velocity (PWV) was measured, PWA with provocative pharmacological tests was performed and IMT measurement of the carotid arteries was conducted in a supine position. Venous blood samples were drawn from the antecubital vein for measurement of plasma ADMA, high-sensitivity C-reactive protein (hsCRP), total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, creatinine and glucose. Height and weight were recorded and BMI was calculated. Waist and hip circumferences were measured and the waist-to-hip ratio was calculated.
Blood sample collection
Venous blood samples were obtained from each patient. Blood samples were centrifuged within 15 min of collection and frozen at −70°C until analysis. ADMA was determined from plasma by using a validated ELISA assay (DLD Diagnostika, Hamburg, Germany) as described previously (Citation8). The other analyses were performed by standard laboratory methods using certified assays in the local clinical laboratory. Renal function was assessed using estimated glomerular filtration rate (eGFR), calculated with the abbreviated MDMR equation (Citation9).
Hemodynamic measurements
Brachial blood pressure was measured in a sitting position in the non-dominant arm as a mean of three consecutive measurements at 5-min intervals using a validated oscillometric technique (OMRON M4-I; Omron Healthcare Europe BV®, Hoofddorp, Netherlands). Brachial pulse pressure was calculated as the difference between brachial systolic and diastolic blood pressure.
Aortic PWV and endothelial function were measured using a Sphygmocor apparatus with the accordant software (SCOR Px, version 7.1; Atcor Medical®, Sydney, Australia). The radial artery pressure waveform was recorded by applanation tonometry with a high fidelity micromanometer (SPT-301B, Millar Instruments®, Houston, TX, USA). The aortic pressure waveform was obtained from the radial pressure waveform using the validated generalized transfer function, from which central systolic and diastolic blood pressure, central pulse pressure and augmentation index (AIx) were calculated. Augmentation index (AIx) was identified as the difference between the second and the first systolic peaks of the central arterial waveform, expressed as the percentage of central pulse pressure. As AIx is dependent on heart rate, it was corrected for a heart rate of 75 beats/min (AIx@75). The time of return of the reflected wave (Tr) was the time from the beginning upstroke of the derived aortic systolic pressure waveform to the inflection point.
Measurements of PWA were done at baseline (two recordings), and at every 5 min for 25 min after 400 μg of salbutamol inhalation with a spacer (GlaxoSmithKline®, Evreux, France). After that, a 500-μg tablet of nitroglycerine (Nycomed®, Roskilde, Denmark) was placed under the tongue for 3 min and AIx@75 was measured at every 3–5 min for 20 min. The greatest differences from baseline AIx@75 with both pharmacological interventions was used to assess EDV and endothelium-independent vasodilation, respectively (Citation6,Citation10).
Aortic PWV (two recordings) was measured by sequentially recording the ECG-gated carotid and femoral artery waveforms, as described previously (Citation11).
Carotid artery IMT
IMT scans of the common carotid artery were performed by a specialist (PM) using an ultrasound device (Sonos7500, Philips Medical Systems, Inc.®, Andover, MA, USA) with a 12-MHz transducer and videotaped on a super-VHS VCR for further analysis. Longitudinal images of distal 1 cm of the left and right common carotid arteries at the far wall and near wall were measured in three projections (anterolateral, lateral and posterolateral). The scans were measured with the Prosound software (Caltech®, Pasadena, CA, USA) by an independent technician at the University of Eastern Finland, Finland. The average of mean IMT was used in analysis.
Statistical analysis
Statistical analysis was performed with the Statistica software (version 8; Statsoft®, Tulsa, OK, USA). All data were tested for normality using the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± standard deviation; non-normally distributed data are presented as the median with the interquartile range. Since the distributions of hsCRP and triglycerides were skewed, they were log-transformed for analysis to improve normality. Correlations between the variables were examined using univariate linear regression and stepwise multiple regression analyses. The predictors for stepwise correlation analysis were selected on the basis of simple correlation analysis and from among the variables known or likely to be associated with the dependent variable. Unpaired Student's t-test was used to compare ADMA levels in smokers and non-smokers. Significance was defined as two-sided p < 0.05.
Results
The clinical characteristics of the study group are summarized in . Ten (12.5%) study subjects were smokers. Hormone replacement therapy was not used by any female patient. One female patient received oral contraceptives as concomitant therapy.
Table I. Baseline characteristics of the study subjects.
ADMA levels were significantly decreased in smokers (0.51 μmol/l) compared with non-smokers (0.65 μmol/l) (p = 0.01). ADMA was significantly correlated with EDV (r = −0.26; p = 0.02) () and IMT (r = 0.32; p = 0.007) (). No correlation was detected between ADMA and AIx, aortic PWV, central and brachial blood pressure values or eGFR. IMT was positively correlated with age (r = 0.39; p = 0.001). There was no correlation between EDV and IMT or EDV and age. A multiple regression model was constructed with ADMA as the dependent variable. All known or likely determinants of ADMA were entered into the model. Female gender and IMT correlated positively with ADMA, and there was an inverse correlation with EDV and total cholesterol levels, but not smoking status (R2 = 0.30; p < 0.001) ().
Figure 1. Correlation between asymmetric dimethylarginine (ADMA) and endothelium-dependent vasodilation (EDV).
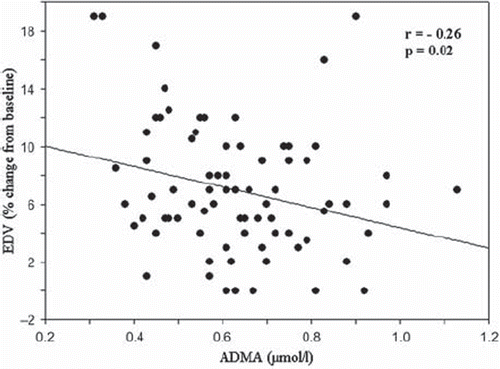
Figure 2. Correlation between asymmetric dimethylarginine (ADMA) and carotid intima-media thickness (IMT).
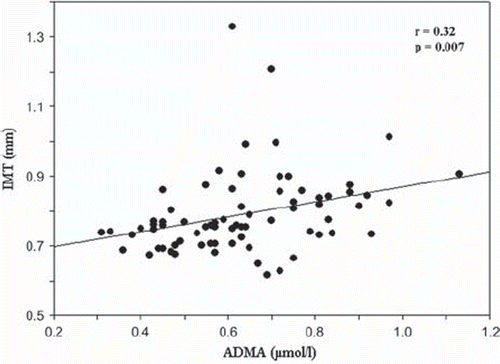
Table II. Results of multiple regression analysis with asymmetric dimethylarginine (ADMA) as the dependent variable.
Discussion
This study shows that ADMA, a powerful inhibitor of e-NOS, is independently associated with EDV measured by PWA with pharmacological tests in hypertensive patients. In addition, ADMA was associated with IMT, which highlights further its role as a marker of vascular structure impairment in hypertension. According to our knowledge, this is the first study where PWA with pharmacological provocation tests has been performed in hypertension patients.
In hypertensive patients, endothelial dysfunction is associated with cardiovascular events (Citation12). It has been shown that PWA with pharmacologic tests allows assessment of endothelial function (Citation6). The advantage of assessment of endothelial function with the use of PWA is that it embraces global endothelial function, i.e. conduit and resistance vessels. Flow-mediated vasodilation and venous occlusion plethysmography, however, are used to assess only conduit and resistance vessel endothelial function, respectively (Citation13).
In the present study, ADMA was independently and inversely associated with EDV in hypertensive patients. Our finding is in accordance with a previous study where venous occlusion plethysmography was used for EDV assessment (Citation4). As reduction of EDV occurs in the preclinical stage of atherosclerosis (Citation14), ADMA serves as an early marker of vascular impairment in hypertension. ADMA contributes to atherosclerosis and is considered a good marker of endothelial dysfunction (Citation15). Moreover, ADMA predicts all-cause mortality in the community (Citation16) and cardiovascular events in various clinical conditions (Citation17–19). It has been suggested that the vasodilating beta-blocker nebivolol reduces endothelial dysfunction through its lowering effect on ADMA (Citation20). Thus, ADMA could potentially be a target marker in hypertension.
Aortic PWV is increased in hypertension patients (Citation21) and is associated with cardiovascular risk (Citation7). In our study, ADMA and EDV were not correlated with aortic PWV. Our findings are concordant with the PREVENCION study, which confirmed that ADMA is associated with IMT and several traditional cardiovascular risk factors but not with arterial stiffness (Citation22). To date, the correlation between EDV and aortic PWV has only been shown in middle-aged healthy persons (Citation13) and in isolated systolic hypertension (ISH) patients (Citation23). This could be explained by the fact that in these patients ISH results from increased arterial stiffness, i.e. aortic PWV, which differs considerably from aortic PVW in non-ISH hypertensives. The mean aortic PWV in our study subjects was 7.4 m/s, which is considerably lower than it is in ISH patients (9.65 m/s) (Citation23). However, owing to the very strict inclusion criteria of the present study, our patients were at low or moderate total cardiovascular risk, which may also explain the normal range of aortic PWV.
The present study did not reveal significant correlations between the values of central/brachial blood pressure and ADMA. In addition, AIx, aortic PWV and mean arterial pressure, the major determinants of central blood pressure, were not correlated with ADMA. Our finding is in accordance with previous evidence that ADMA is not correlated with brachial blood pressure (Citation22). Earlier experimental studies have shown that the elevated plasma concentration of ADMA increases mean arterial pressure (Citation24,Citation25). In the clinical setting, the concentration of ADMA in plasma is lower because ADMA is concentrated intracellularly and is rapidly eliminated by dimethylarginine dimethylaminohydrolase (DDAH).
In this study, we showed that ADMA is associated with an early marker of vascular disease, IMT, in hypertension patients. A large body of evidence shows that IMT is significantly related to incident cardiovascular events, both coronary events and stroke (Citation7). It has been shown previously that ADMA is associated with increased IMT in healthy subjects (Citation26). In the present study, IMT was positively correlated with age, which is in accordance with the findings of other similar studies. We confirmed that EDV and IMT are not correlated, which suggests that they measure different aspects and stages of early atherosclerosis (Citation27). It is possible that endothelial dysfunction as the earliest event in the process of lesion formation precedes structural changes in the arteries; thus increase in IMT could be a consequence of endothelial dysfunction (Citation28). As common carotid IMT reflects mostly vascular medial hypertrophy (Citation7), we can suggest that atherosclerosis and arteriosclerosis are inter-related in hypertension patients.
Interestingly, ADMA was increased in non-smokers compared with smokers in our study. Data about the relationship between ADMA and smoking status are conflicting (Citation29). Smoking has been associated with decreased levels of ADMA in healthy individuals and in high-risk patients (Citation30,Citation31). In our study, this effect could be related to light smoking (less than 10 cigarettes a day). Furthermore, to minimize the acute effect of smoking on arterial stiffness and several biomarkers, the patients discontinued smoking at least 12 h before the study. There is evidence that smoking enhances the expression of DDAH, which may decrease ADMA levels (Citation30). It has also been suggested that endothelial dysfunction caused by tobacco smoke is probably not related to ADMA (Citation29).
There are some limitations to the present study. The relatively limited number of subjects confined the inclusion and exclusion criteria of hypertension patients in our study. As the study is cross-sectional, it was not within our competence to evaluate causality between ADMA and endothelial dysfunction or IMT in hypertension patients. We did not use a special control group in addition to hypertension patients. Thus, the findings of the present study should not be directly generalized. Additional investigations are needed to confirm our observations in healthy subjects.
In conclusion, ADMA is associated with endothelial dysfunction as assessed by PWA and IMT in hypertension patients. Furthermore, PWA combined with provocative pharmacological tests is a useful method for assessment of endothelial function. These findings may have clinical importance, as cardiovascular risk evaluation cannot be fully explained by traditional risk factors. Further research is required to assess the prognostic value of plasma ADMA level reduction associated with antihypertensive therapy.
Acknowledgements
This study was supported by the Estonian Science Foundation grants Nos. 7480 and 8273, by target financing Nos. 0180105s08 and SF0180001s07, and partly by the European Union through the European Regional Development Fund. We would like to thank Eva-Brit Mölder for the assistance in the study and Jarmo Tiikainen for IMT image analysis. We would also like to thank Ester Jaigma for linguistic revision of the manuscript and all patients who participated in the study.
Conflict of interest: The authors declare no conflict of interest.
References
- World Health Organization. Mortality and Burden of Disease Attributable to Selected Major Risks. 2009; http://www.who.int/healthinfo/global_burden_disease/
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32.
- Panza JA, Quyyumi AA, , Brush JE JrEpstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27.
- Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, . Asymmetric dimethylarginine, l-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–523.
- Calver A, Collier J, Leone A, Moncada S, Vallance P. Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J Hum Hypertens. 1993;7:193–194.
- Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, . Pulse-wave analysis: Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–152.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 ESH-ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25: 1751–1762.
- Kals J, Kampus P, Kals M, Teesalu R, Zilmer K, Pulges A, . Arterial elasticity is associated with endothelial vasodilatory function and asymmetric dimethylarginine level in healthy subjects. Scand J Clin Lab Invest. 2007;67: 536–544.
- , Brosius FC 3rdHostetter TH, Kelepouris E, Mitsnefes MM, Moe SM, Moore MA, . Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease. Circulation. 2006;114:1083–1087.
- Kals J, Kampus P, Kals M, Pulges A, Teesalu R, Zilmer M. Effects of stimulation of nitric oxide synthesis on large artery stiffness in patients with peripheral arterial disease. Atherosclerosis. 2006;185:368–374.
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockroft JR, . Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084.
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, . Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104: 191–196.
- McEniery CM, Wallace S, Mackenzie IS, McDonnell B, , YasminNewby DE, . Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608.
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994; 24:1468–1474.
- Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20:2032–2037.
- Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, . Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600.
- Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J. 2003;24:1912–1919.
- Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, . Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet. 2001;358: 2113–2117.
- Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, . Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–2540.
- Pasini AF, Garbin U, Stranieri C, Boccioletti V, Mozzini C, Manfro S, . Nebivolol treatment reduces serum levels of asymmetric dimethylarginine and improves endothelial dysfunction in essential hypertensive patients. Am J Hypertens. 2008;21:1251–1257.
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15.
- Chirinos JA, David R, Bralley JA, Zea-Díaz H, Muñoz-Atahualpa E, Corrales-Medina F, . Endogenous nitric oxide synthase inhibitors, arterial hemodynamics, and subclinical vascular disease: The PREVENCION Study. Hypertension. 2008;52:1051–1059.
- Wallace SM, , YasminMcEniery CM, Mäki-Petäjä KM, Booth AD, Cockcroft JR, . Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007;50:228–233.
- Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, . Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177.
- Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, . Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459.
- Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, . Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation. 1999;99:1141–1146.
- Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol. 2005;45: 1980–1986.
- Lundman P, Eriksson MJ, Stühlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol. 2001;38:111–116.
- Sobczak A, Goniewicz ML, Szoltysek-Boldys I. ADMA and SDMA levels in healthy men exposed to tobacco smoke. Atherosclerosis. 2009;205:357–359.
- Maas R, Schulze F, Baumert J, Löwel H, Hamraz K, Schwedhelm E, . Asymmetric dimethylarginine, smoking, and risk of coronary heart disease in apparently healthy men: Prospective analysis from the population-based Monitoring of Trends and Determinants in Cardiovascular Disease/Kooperative Gesundheitsforschung in der Region Augsburg study and experimental data. Clin Chem. 2007; 53:693–701.
- Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism. 2004;53:1574–1579.