Abstract
It has been reported that the number of circulating endothelial progenitor cells (EPCs) reflects the endogenous vascular repair ability, with the EPCs pool declining in the presence of cardiovascular risk factors. However, their relationship with hypertension and the effects of anti-hypertensive treatment remain unclear. We randomized 29 patients with mild essential hypertension to receive barnidipine up to 20 mg or hydrochlorothiazide (HCT) up to 25 mg. Circulating EPCs were isolated from peripheral blood at baseline and after 3 and 6 months of treatment. Mononuclear cells were cultured with endothelial basal medium supplemented with EGM SingleQuots. EPCs were identified by positive double staining for both FITC-labeled Ulex europaeus agglutinin I and Dil-labeled acethylated low-density lipoprotein. After 3 and 6 months of treatment, systolic and diastolic blood pressure (BP) were significantly reduced. No difference was observed between drugs. An increase in the number of EPCs was observed after 3 and 6 months of anti-hypertensive treatment (p < 0.05). Barnidipine significantly increased EPCs after 3 and 6 months of treatment, whereas no effect was observed with HCT. No statistically significant correlation was observed between EPCs and clinical BP values. Our data suggest that antihypertensive treatment may increase the number of EPCs. However, we observed a different effect of barnidipine and HCT on EPCs, suggesting that, beyond its BP lowering effect, barnidipine may elicit additional beneficial properties, related to a healthier vasculature.
Introduction
Essential hypertension has been extensively reported to cause endothelial dysfunction and damage, characterized by unbalanced vasodilation and vasoconstriction, increased oxidative stress, vascular inflammation, alteration of prothrombotic and fibrinolytic pathways, abnormal smooth muscle cell proliferation and impaired repair mechanisms (Citation1,Citation2). All these factors contribute to vascular disease, to the development and progression of atherosclerosis and to the onset of cardiovascular events (Citation3–5). Therefore, integrity of the endothelial monolayer plays a crucial role in the prevention of hypertension and atherosclerosis. Recently, it has been demonstrated that the number of circulating endothelial progenitor cells (EPCs) reflects the endogenous ability to repair endothelial and vascular damage. The EPC pool declines in the presence of cardiovascular risk factors (Citation6,Citation7). EPCs are recruited from the bone marrow to areas of endothelial injury, where they differentiate and promote revascularization, restoring the integrity of the monolayer of the endothelium (Citation7).
Numerical and functional impairment of EPCs are inversely related to cardiovascular risk factors and increased atherosclerotic disease risk (Citation8), and they have been linked to endothelial dysfunction (Citation9,Citation10), and to increased cardiovascular morbidity and mortality (Citation11,Citation12).
In recent years, it has been suggested that pharmacological agents used to correct cardiovascular risk factors, such as statins (Citation13) and some antihypertensive drugs (Citation14–16), may positively influence EPCs. Most of these drugs have been already demonstrated to improve endothelial function in hypertension and other vascular diseases. In particular, drugs acting on the renin–angiotensin system, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers have been reported to improve EPC number and function (Citation14,Citation15) and this effect seems related more to a reduction of oxidative stress than to a reduction in blood pressure (BP). Similarly, it has been recently shown that also calcium-channel blockers may improve EPC number and functional activity (Citation16). However, the relationship between EPC number and the presence of hypertension as well as the effect of anti-hypertensive treatment on EPC remain still controversial (Citation17).
The aim of this study was to investigate the effects of a relatively new dihydropyridinic calcium-channel blocker, barnidipine, in comparison with a thiazide diuretic, hydrochlorothiazide, on EPCs numbers in patients with mild essential hypertension.
Patients and methods
Twenty-nine patients with mild to moderate hypertension were randomized to receive barnidipine (up to 20 mg per day) or hydrochlorothiazide (up to 25 mg per day) for 6 months. Patients with previous cardiovascular event, clinic or laboratory evidence of heart or renal failure, diabetes mellitus, malignant disease or active inflammation as well as patients previously treated with statins or acetylsalicylic acid were excluded from the study. Three patients were previously treated for not more than 1 year with a calcium-channel blocker; two of them were randomized to receive barnidipine and one hydrochlorothiazide. Previous antihypertensive treatment was withdrawn at least 2 weeks before study entry. Clinic BP was measured by standard mercury sphygmomanometer according to European Society of Hypertension–European Society of Cardiology Guidelines (Citation18) and patients were included in the study if their systolic BP (SBP) ranged between 140 and 179 mmHg and/or their diastolic BP (DBP) ranged between 90 and 109 mmHg in the sitting position.
Venous blood samples were obtained from each patient, after overnight fasting, for standard laboratory tests [total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides and plasma glucose] at baseline and after 3 and 6 months of treatment.
The protocol of the study was approved by the ethics committee of our institution (Medical School, University of Brescia), and informed consent was obtained from each participant. The procedures followed were in accordance with institutional guidelines.
Isolation of EPCs
The number of EPCs was assessed using an in vitro assay using a previously described technique (Citation13,Citation14). In brief, peripheral blood mononuclear cells were isolated from patients’ blood using density gradient centrifugation with Ficoll (Amersham) and seeded on six-well plates coated with human fibronectin (Sigma) in endothelial basal medium (EBM-2; Clonetics) supplemented with growth factors (VEGF, FGF-2, EGF, IGF-1), ascorbic acid, hydrocortisone, gentamicin and 10% fetal bovine serum.
After 7 days in culture, we performed fluorescent chemical detection of the attached cells using 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated LDL (acLDL-DiI, 2.4 mg/ml; Molecular Probes) and fluorescein isothiocyanate-labeled Ulex europaeus agglutinin-1 (10 mg/ml UEA-1; Sigma) for cell staining. Samples were viewed with an inverted fluorescent microscope and counted (cell number/107 plated cells per 1.17 mm2 of area). EPCs were identified by positive double staining for both UEA-1 and acLDL-DiI. DAPI were used to stain the nuclei.
Statistical analysis
Results are expressed as the means±SD. Comparison of continuous variables in the clinical study was performed by a Student paired or unpaired t-test, as appropriate. Relationships between different variables were assessed by calculating Pearson's correlation coefficient (r). The statistical significance was set at the conventional level of 5%. All variables investigated were normally distributed. We estimated that our study had a power of 95%, at a 5% significance level, to detect a difference of 18 EPCs/107 plated cells per 1.17 mm2 of area within or between groups.
Results
Demographic, haemodynamic and humoral characteristics for the patients are summarized in . No baseline difference in age, gender, clinical SBP and DBP, body mass index, fasting blood glucose and humoral indices for cardiovascular risk (including lipid profile) was detected between patients randomized to barnidipine or hydrochlorothiazide. No difference is the smoking habits was present between the two groups and no changes in this risk factor were observed during the relatively short treatment period. No difference in the cardiovascular risk profile was observed between the two treatment groups. None of them had left ventricular hypertrophy or microalbuminuria.
Table I. Demographic characteristics of patients enrolled in the study.
No change was observed in body mass index and in physical activity during the treatment period.
In patients treated with hydrochlorothiazide, a significant increase in LDL-cholesterol levels was observed (p < 0.05) (). Conversely, a significant reduction in LDL-cholesterol was detected in patients treated with barnidipine after 3 and 6 months of treatment (p < 0.05) (). Hydrochlorothiazide induced a significant reduction in serum potassium level (p < 0.05, ), which was not present in patients treated with barnidipine. Considering the two treated groups together, clinical SBP and DBP were significantly reduced after 3 and 6 months of treatment. No statistically significant difference was observed between the two drug regimens in the extent of BP reduction ().
EPC number
EPC number at baseline did not differ in the two subgroups of patients receiving either barnidipine or hydrochlorothiazide. Considering the two treated group together, EPC number (expressed as number of EPCs/107 plated cells per 1.17 mm2 of area) resulted increased after 3 months (62.5 ± 43.6, p < 0.05) and 6 months (61.4 ± 40.3, p < 0.05) of treatment compared with baseline (40.9 ± 21.9) ( and ). The difference between groups in terms of changes in EPCs persisted even after correction for baseline BP, cholesterol, glucose and age.
Figure 1. Effect of antihypertensive treatment on endothelial progenitor cell (EPC) number in hypertensive patients. Absolute number of EPCs in hypertensive patients at baseline conditions (black bars) and after 3 months (grey bars) and 6 months (white bars) of antihypertensive therapy in the whole group of hypertensive patients (all patients), in patients treated with barnidipine (BD) and hydrochlorothiazide (HCT). EPCs are expressed as absolute number of EPCs/107 plated cells per 1.17 mm2 of area. Data are presented as means ± SD. *p < 0.05 vs baseline conditions.
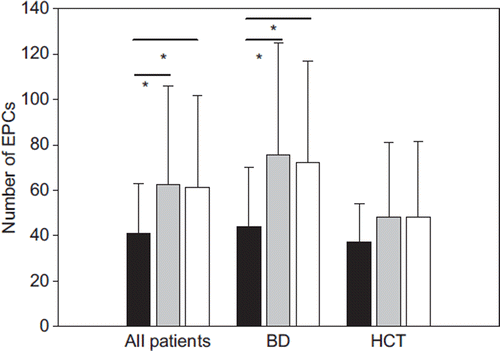
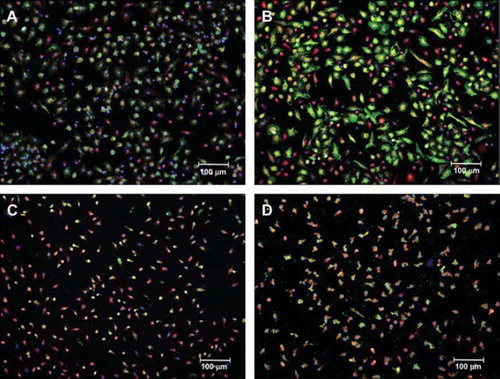
Figure 2. Representative image of endothelial progenitor cells (EPCs). Upper panels show representative images of EPCs at baseline conditions (A) and after a 6-month treatment period (B) with barnidipine (BD). The bottom panels show representative images of EPCs at baseline conditions (C) and after a 6-month treatment period (D) with hydrochlorothiazide (HCT). EPCs were identified by positive double staining for both FITC-labeled Ulex europaeus agglutinin I (green fluorescence) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (red fluorescence). DAPI were used to stain the nuclei (blue fluorescence). *p < 0.05 vs baseline conditions. See Method section for more details.
However, when patients were subdivided according to the drug treatment, the EPC number significantly increased in patients treated with barnidipine after 3 (75.5 ± 49.2, p < 0.05) and 6 months (72.4 ± 44.8, p < 0.05) of treatment, compared with baseline (44.2 ± 26.1). No significant difference was observed in patients treated with hydrochlorothiazide (48.3 ± 32.9 and 48.4 ± 33.0 after 3 and 6 months, respectively, p = 0.35 and p = 0.33) compared with baseline (37.4 ± 16.6) ().
Correlations
No statistically significant correlation was observed between EPC number and clinical SBP or DBP values or total or LDL-cholesterol levels. Finally, no statistically significant correlation was observed between changes in LDL and changes in EPC number.
Discussion
The present study demonstrated an increase of EPC number during effective antihypertensive treatment. In addition, when hypertensive patients were subdivided according to drug treatment, barnidipine appeared more effective in increasing EPC number compared with hydrochlorothiazide, despite a similar BP lowering effect.
The development of hypertension in humans appears to be associated with an impairment of endothelial function (Citation19–22). It has been well established that vascular damage in hypertension may be partly ascribed to alterations of several endothelium-dependent mechanisms. Increased oxidative stress, related to an impaired nitric oxide bioavailability, induced by production of reactive oxygen species, has been associated to an impaired endothelial function in hypertension and atherosclerosis (Citation1,Citation2) and may play a relevant role in the pathogenesis of cardiovascular events (Citation3–5). Several classes of antihypertensive drugs have been shown to improve endothelial function. There is evidence that dihydropyridine calcium-channel blockers and inhibitors of the renin–angiotensin system increase nitric oxide bioavailability and decrease oxidative stress, thus improving endothelial and vascular function in hypertension (Citation20,Citation23–25). In this regard, amlodipine, as well as other calcium-channel blockers, have been reported to possess antioxidant properties (Citation26,Citation27). On the other hand, other antihypertensive agents failed to improve endothelial function despite effective BP reduction, thus suggesting that some antihypertensive agents have beneficial properties beyond their antihypertensive effect (Citation20,Citation24,Citation28,Citation29). Indeed, hydrochlorothiazide has been demonstrated to be ineffective in ameliorating endothelial function and reducing oxidative stress in hypertension experimental model (Citation29). It should also be considered that some drugs, including calcium-channel blockers, such as nifedipine, were demonstrated to be able to improve endothelial function in the microcirculation, when endothelial-dependent responses were evaluated either by micromyographic approaches (Citation30) or by the isolated forearm plethysmographic technique (Citation27). Conversely, no effect was observed on flow-mediated dilatation of the brachial artery (Citation31), thus suggesting the presence of some heterogeneity in this regard, probably in relation to the fact that different indices probably explore different aspects of endothelial function. While alterations in flow-mediated dilatation of the brachial artery point toward the presence of endothelial dysfunction, EPC number is an indicator of the presence of more severe endothelial damage. In fact, EPC number was demonstrated to be related to other indicators of endothelial damage, such as von Willebrand factor circulating levels (Citation32).
In the present study, we found that barnidipine, but not hydrochlorothiazide, induced a significant increase also in EPC number. Several studies have investigated the association between EPCs and hypertension, as well as the effects of antihypertensive treatment. In patients with coronary artery disease, an inverse correlation between arterial pressure and the number and the migratory capacity of EPCs has been reported (Citation8,Citation12). The presence of hypertension was significantly correlated with a reduction in circulating EPCs also in otherwise healthy subjects (Citation8,Citation9), although no differences were observed in a different study (Citation17).
Bone marrow-derived EPCs play a critical role in maintaining endothelial function by contributing to re-endothelialization and neovascularization (Citation6,Citation7), and impaired mobilization or depletion of these cells may contribute to endothelial dysfunction and cardiovascular disease progression. Several drugs may increase the number and the functional activity of EPCs (Citation13,Citation14,Citation33). Angiotensin receptor antagonists have been reported to increase the number of EPCs in diabetic patients, and this effect is independent from their BP-lowering action (Citation14). Indeed, angiotensin II may accelerate the EPC senescence and may impair EPC proliferation through gp91phox-mediated increase in oxidative stress, and this effect was inhibited by treatment with the angiotensin II type 1 receptor blocker valsartan (Citation33). Recently, the calcium-channel blocker nifedipine has been demonstrated to improve endothelial function, to promote differentiation and/or proliferation of cultured EPCs and to increase the ability of EPCs to resist to oxidant stress in patients with mild essential hypertension after a 4-week treatment period (Citation16). In fact, nifedipine has been shown to increase nitric oxide production in hypertensive subjects and in cultured endothelial cells (Citation34). On the contrary, the diuretic hydrochlorothiazide was demonstrated to be unable to increase the number of EPCs, at least in a mouse model of angiotensin II dependent hypertension (Citation35), thus supporting our observation.
Barnidipine is a new dihydropyridine calcium antagonist with long-lasting vasodilator properties (Citation36), and possibly antioxidant effects (Citation37). The different effect of barnidipine compared with hydrochlorothiazide observed in our study may have several explanations. First of all, the improvement of endothelial function and EPCs might be ascribed to the potential antioxidant properties of this agent. This hypothesis is in agreement with the observation of no further increase in the effect from 3 to 6 months, since antioxidant effects occur usually very rapidly. In our study, LDL-cholesterol, which is known to be associated to altered endothelial function (Citation38), was reduced during barnidipine treatment already after 3 months. Although several studies report that calcium antagonists, including barnidipine (Citation37) are metabolically neutral (Citation39,Citation40), other studies have shown a beneficial effect of calcium antagonists on lipid profile. A previous report has demonstrated an increase in HDL-cholesterol during treatment with calcium antagonists (Citation41). A significant increase in HDL-cholesterol and HDL-cholesterol/LDL-cholesterol ratio and decrease in total cholesterol/HDL-cholesterol ratio have also been recently reported after treatment with amlodipine (Citation42). Our findings were therefore in keeping with few previous reports that examined the effects of various antihypertensive agents on serum lipid parameters, although the action of calcium antagonists on plasma lipid levels remains controversial. In fact, the decrease of LDL-cholesterol observed in patients treated with barnidipine might also have been a chance finding.
On the other hand, in the group of patients treated with hydrochlorothiazide, we observed a significant increase in LDL-cholesterol level compared with baseline, which might have contributed, at least in part, to the lack of significant changes in EPC number observed with hydrochlorothiazide. Previous studies have reported that diuretic therapy has been associated with increase in serum total cholesterol and LDL-cholesterol (Citation43). The underlying mechanisms remain uncertain, even though a reduced insulin sensitivity and hyperinsulinemia has been suggested to be involved (Citation44).
In our study, no statistically significant correlation was observed between changes in lipid profile and changes in EPC number. This result is in keeping with recent work by Schmidt-Lucke et al. (Citation45), who found that cholesterol lowering per se is not responsible for increasing EPC numbers.
Our study has some limitations. The number of patients enrolled is relatively small. The precise mechanism by which barnidipine may influence EPCs has not been investigated in the present study, and remains elusive. The increase of EPC number with barnidipine observed in our patients could be a result of either EPC mobilization from the bone marrow or reduced EPC senescence through an improvement of endothelial function and oxidative stress. Thus, further studies are needed in this regard, in particular focusing also on telomere shortening (Citation46), although effects of lipids, inflammation and oxidative stress remain good possible explanations. Finally, there is no general agreement about the best methodological approach to EPC count, since no gold standard is acknowledged. However, the methods used in the present study seems to provide a reasonable accuracy, specificity and sensitivity (Citation13,Citation14,Citation44).
In conclusion, EPC number predicts the occurrence of cardiovascular events and cardiovascular death, at least in patients with coronary artery disease, as well as in healthy control subjects (Citation11,Citation12). Hence, EPC number may represent a potential drug target in hypertension; in this regard, the calcium-channel blocker barnidipine may be considered a useful therapeutic option.
Acknowledgement
This study was partly financed by the European Community, Sixth Framewok program “InGenious HyperCare”.
Conflict of interest: No financial or other relations that could lead to a conflict of interest.
References
- Ross R. The pathogenesis of atherosclerosis – An update. N Engl J Med. 1986;314:488–500.
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295.
- Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;1111:363–368.
- Drexler H. Endothelial dysfunction: Clinical implications. Prog Cardiovasc Dis. 1997;39:287–324.
- Widlansky MR, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, . Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, . Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228.
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, . Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7.
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, . Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
- Quyyumi AA, Hill JM. Circulating endothelial progenitor cells as novel biological determinants of vascular function and risk. Can J Cardiol. 2004;20 Suppl B:44B–48B.
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, . Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987.
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, . Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007.
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, . HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397.
- Bahlmann FH, de Groot K, Mueller OH, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells – A new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension. 2005;45:526–529.
- Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2004;18:203–209.
- Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, . Nifedipine improves endothelial function: Role of endothelial progenitor cells. Hypertension. 2008;52:491–498.
- Delva P, Degan M, Vallerio P, Arosio E, Minuz P, Amen G, . Endothelial progenitor cells in patients with essential hypertension. J Hypertens. 2007;25:127–132.
- Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053.
- Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Tiberio G, . Endothelial dysfunction in hypertension is independent from the etiology and from vascular structure. Hypertension. 1998;312:335–341.
- Muiesan ML, Salvetti M, Monteduro C, Rizzoni D, Zulli R, Corbellini C, . Effect of treatment on flow-dependent vasodilation of the brachial artery in essential hypertension. Hypertension. 1999;33(Part II):575–580.
- Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27.
- Iiyama K, Nagano M, Yo Y, Nagano N, Kamide K, Higaki J, . Impaired endothelial function with essential hypertension assessed by ultrasonography. Am Heart J. 1996;132:779–782.
- Mizuno Y, Jacob RF, Mason RP. Effects of calcium channel and renin–angiotensin system blockade on intravascular and neurohormonal mechanisms of hypertensive vascular disease. Am J Hypertens. 2008;21:1076–1085.
- Schiffrin EL, Pu Q, Park JB. Effect of amlodipine compared to atenolol on small arteries of previously untreated essential hypertensive patients. Am J Hypertens. 2002, 15(2 Part 1): 105–110.
- Taddei S, Virdis A, Ghiadoni L, Uleri S, Magagna A, Salvetti A. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30:1606–1612.
- Ganafa AA, Walton M, Eatman D, Abukhalaf IK, Bayorh MA. Amlodipine attenuates oxidative stress-induced hypertension. Am J Hypertens. 2004;17:743–748.
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Favilla S, Pompella A, . Restoration of nitric oxide availability after calcium antagonist treatment in essential hypertension. Hypertension. 2001;37:943–948.
- Wassmann S, Hilgers S, Laufs U, Böhm M, Nickenig G. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia associated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1208–1212.
- Zhou MS, Schulman IH, Jaimes EA, Raij L. Thiazide diuretics, endothelial function, and vascular oxidative stress. J Hypertens. 2008;26:494–500.
- Schiffrin EL, Deng LY. Structure and function of resistance arteries of hypertensive patients treated with a beta blocker or a calcium channel antagonist. J Hypertens. 1996;14:1237–1244.
- Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, . Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–1286.
- Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079.
- Imanishi T, Hano T, Nishio I. Angiotensin II potentiates vascular endothelial growth factor-induced proliferation and network formation of endothelial progenitor cells. Hypertens Res. 2004;27:101–108.
- Berkels R, Egink G, Marsen TA, Bartels H, Roesen R, Klaus W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension. 2001;37:240–245.
- Yu Y, Fukuda N, Yao EH, Matsumoto T, Kobayashi N, Suzuki R, . Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens. 2008;21:72–77.
- Malhotra HS, Plosker GL. Barnidipine. Drugs. 2001;61:989–996.
- Spirou A, Rizos E, Liberopoulos EN, Kolaitis N, Achimastos A, Tselepis AD, . Effect of barnidipine on blood pressure and serum metabolic parameters in patients with essential hypertension: A pilot study. J Cardiovasc Pharmacol Ther. 2006;11:256–261.
- Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 suppl 1):III15–III19.
- Kasiske BL, Ma JZ, Kalil RSN, Louis TA. Effects of antihypertensive therapy on serum lipids. Ann Intern Med. 1995; 122:133–141.
- Brook RD. Mechanism of differential effects of antihypertensive agents on serum lipids. Curr Hypertens Rep. 2000;2:370–377.
- Leonetti G. Effects of nilvadipine and amlodipine in patients with mild to moderate essential hypertension: A double blind, prospective, randomized clinical trial. Curr Med Res Opin. 2005;21:951–958.
- Nandeesha H, Pavithran P, Madanmohan T. Effect of antihypertensive therapy on serum lipids in newly diagnosed essential hypertensive men. Angiology. 2009;60:217–220.
- Neutel JM. Metabolic manifestations of low-dose diuretics. Am J Med. 1996;101:71S–82S.
- Lithell H. Hypertension and hyperlipidemia. Am J Hypertens. 1993;6:303S–308S.
- Schmidt-Lucke C, Fichtlscherer S, Rössig L, Kämper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010;211:249–254.
- Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvàth T, . Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension. Relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397.