Abstract
Introduction. The aim of this study was to assess blood flow in the vessels of the eyeball and changes in the optic nerve in patients with arterial hypertension and primary open-angle glaucoma. Material and Methods. The patients were divided into groups: 1 (night blood pressure, BP, fall, NBPF, not more than 10%; non-dippers); 2 (NBPF 10–15%, dippers) and 3 (NBPF>15%; extreme dippers). Results. In the group of dippers and extreme dippers, perfusion pressure was significantly lower than that in the non-dippers group, there was reduced thickness of the nerve fibers and a greater decrease in the visual field. Significant relationships between peak systolic, end-diastolic flow in the ophthalmic and central retinal arteries and night perfusion pressure, thickness of nerve fibers, and a loss of visual field were observed. Conclusion. In patients with glaucoma and well-controlled hypertension, a nocturnal BP fall of more than 10% is associated with a greater visual field defect and greater degeneration of the optic nerve fibers. Low minimum diastolic pressure and the level of nocturnal BP fall, but not the absolute value of average arterial BP at night, should be included in the group of specific risk factors in patients with hypertension and open-angle glaucoma. These findings also suggest avoiding excessive lowering of BP at night in this group.
Introduction
Arterial hypertension (AH) and glaucoma occur simultaneously in an aging population. In previous studies it has been shown that both AH and low pressure at night are important vascular risk factors for open-angle glaucoma, the so-called simple glaucoma (Citation1–3) This is the most common form of the disease encountered in developed countries, where it constitutes over 50% of all cases (Citation4). Primary open-angle glaucoma (POAG) is defined as chronic, progressive optic neuropathy with the characteristic features of anatomical and functional damage. Increased intraocular pressure (IOP) and other, as yet unknown, factors cause the damage, namely the characteristic atrophy of the optic nerve and retinal ganglion cells and their axons. Two large multi-centered trials were designed to evaluate whether the treatment of ocular hypertension prevents or delays the development of open-angle glaucoma: the Ocular Hypertension Treatment Study (OHTS) and the European Glaucoma Prevention Study (EGPS). Higher baseline IOP was a risk factor in OHTS (hazard ratio: 1.10, 95% confidence interval [CI] 1.04–1.17) in EGPS (hazard ratio: 1.18, 95% CI 1.06–1.31) (Citation5,Citation6).
It has been proved that in POAG autoregulation disorders occur and consequently, intensification of fluctuations in IOP can determine the progress of optic nerve damage (Citation7). It follows that physiological changes in the circadian rhythm of blood pressure (BP) may further aggravate ischemia of the optic nerve, among others, because of the fluctuations in ocular perfusion pressure (OPP) that are derived from the IOP and mean arterial pressure (MAP) (Citation8). This means that the situation may be even more complicated when patients with POAG also suffer from hypertension and require hypotensive therapy.
In the ophthalmic literature, it has been pointed out that both the physiological decrease in systemic pressure during sleep, as well as an excessive hypotensive effect of drugs used to treat the AH, can lead to a secondary reduction of perfusion in the vessels of the eyeball and orbit, chronic ischemia of the optic nerve and the development of neuropathy of that nerve (Citation9–13). From a theoretical point of view, perfusion abnormalities in the arteries of the eyeball may be associated with a reduction of BP at night below the value that allows effective self-regulation of intraocular circulation, or with an excessive diurnal–nocturnal pressure difference (the extreme dipper phenomenon) even if the pressure values at night are considered normal (Citation14–16). The impact of nocturnal and circadian changes in BP on blood flow within the arteries of the eyeball, and any possible progression of lesions within the optic nerve, in patients with POAG and AH have not been analyzed so far.
Therefore, the aim of this paper was to assess the impact of different types of circadian rhythm of BP and the nocturnal level of that pressure in relation to the stage of optic nerve neuropathy, and on the characteristics of blood flow in selected arteries of the eyeball and orbit of patients with both POAG and AH.
Material and Methods
Material
The study was conducted between 2007 and 2009 on a group of 69 patients with POAG (according to the European Glaucoma Society classification) and treated, controlled hypertension. In the group, there were 49 females and 20 males, whose mean age was 55.4±15.9 years. Gonioscopy confirmed an open-angle without disturbance of its structure in all the patients. Corrected visual acuity in the group was ≥0.5 with the axial length of the eyeball between 22 and 24.5±1.7 mm and the error of refraction ±4 dioptres. Mean IOP in the group was 16.4±2.49 mmHg. In the treatment of glaucoma, three groups of IOP-lowering drugs were used: beta-blockers, carbonic anhydrase inhibitors and prostaglandin analogues. Patients suffering from eye diseases other than glaucoma, diabetes, ischemic heart disease or ischemic peripheral vascular disease, thyroid diseases, autoimmune diseases, renal and cardiac failure or stroke were not enrolled in the study. To preclude flow disturbances of cardiovascular origin unconnected with systemic BP, echocardiography and Doppler ultrasound of the carotid and vertebral arteries was performed using the Vivid 4 GE, cardiovascular ultrasound system (Transducers: 3S – Cardiac Sector, 7L – Linear).
All the patients met the criteria of effective BP control according to the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) 2007. This meant that the average BP calculated on the basis of at least two visits at a consultation office were at a level <140/90 mmHg and the average BP, calculated on the basis of measurements made at home, were <135/85 mmHg. In the whole group of patients with AH, 55% were taking one hypotensive preparation, 27.5% were taking two preparations and 17.5% were being treated with three drugs from the following groups: diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, Ca-blockers and sartans. The study was approved by the Medical Ethics Committee of Poznan University of Medical Sciences and written informed consent was obtained from all participants.
Methods
A full medical history was taken from all the patients in the study population. All underwent a physical examination, including measuring the BP three times after a 15-min rest in a sitting position using an automatic sphygmomanometer (OmronMX2 Basic, UK). On the same day, the patients had BP monitors (2430TM A&D Company, Japan) placed for 24-h ambulatory BP monitoring (ABPM), and were sent to the eye clinic, where they were subjected to a standard ophthalmological examination. Additionally, in all patients, the retinal nerve fiber layer (RNFL) was measured by means of a Stratus OCT (Carl Zeiss Meditec, Germany). The measurements involved using the Fast RNFL 3.4-mm scan protocol. The length of the scan was 10.87 mm. This examination was aimed at diagnosing the loss of fibers in the temporal quadrants, upper and lower, and at measuring the average total thickness of the fibers in µm. The evaluation of visual field defects (VFD) was performed using an automated perimeter apparatus (MEDMONDT M600W, USA). The following settings were chosen: test type (glaucoma), fixation loss (not higher than 10%), visual field loss diagnosis in a gray scale (from 0 to >27) and numerically in dB. The test ended in indicating the percentage loss in the field of vision. In the event of obtaining false negative/positive responses of more than 10%, the tests were rejected.
Examinations of visual evoked potentials (Pattern VEP 60’/15’) were also carried out, using a RETI-port 32 device (USA) and DTL Thread electrodes with a narrow pupil (2–3 mm). The examinations resulted in a latency of P100 (m/s) and amplitude N75-P100 (µV) estimation in four canals from the right and left eye (R1 1.0°, R1 0.15 min, L1 1.0°, L1 0.15 min).
IOP measurements were also made several times during the day using Goldmann applanation tonometry. These were recorded at 09:00, 12:00, 17:00 (daytime measurements – IOPd) and at 23:00, 06:00 (night-time measurements – IOPn). Doppler ultrasound was used to examine the retrobulbar vessels in all the patients. These examinations involved the use of the Aloka Alpha 10 system with D-eFLOW and a 7.5–13-MHz frequency range transducer. They were carried out with a patient in a supine position after 5 min of rest (0° gaze position of the eyeball and 35–45° correction of the angle waves). The ophthalmic artery (OA), near the branching of the central retinal artery (CRA) (at a depth of 0–3 mm from the optic disc), and the short posterior ciliary arteries (SPCA) (1–3 mm from the nasal optic nerve head, medially and laterally) were all examined. The following parameters of flow were examined: peak systolic velocity (PSV), end-diastolic velocity (EDV) and the vascular resistance index (RI), which was calculated using the formula: RI =(PSV−EDV)/PSV. In the ABPM examination, the hours 08:00–22:00 were considered the period of daily activity, while the hours between 22:00 and 08:00 were considered the period of sleep. Measurements were taken every 30 min throughout the day and night. Only those records in which we obtained over 90% of valid measurements were accepted for the final statistical analysis.
Twenty-four-hour mean systolic BP (mean SBP), 24-h mean diastolic BP (mean DBP), minimum systolic BP (SBPmin), minimum diastolic BP (DBPmin), diurnal mean arterial pressure (MAPd) and nocturnal mean arterial pressure (MAPn) were all analyzed. The MAP was calculated using the formula: MAP =DBP +1/3 (SBP−DBP). On the basis of the data obtained in the ABPM, we estimated the amount of BP reduction in the night (that is the nocturnal BP fall, NBPF), according to the formula: NBPF (%) =MAPd−MAPn/MAPd ×100%. Additionally, on the basis of the same ABPM data, we calculated diurnal and nocturnal OPP in the vessels of the eyeball and the orbit as follows:
Diurnal OPP − OPPd = 2/3 MAPd − IOPd
Nocturnal OPP − OPPn = 2/3MAPn − IOPn.
The results obtained in the study were analyzed for the whole group and after dividing the patients into three subgroups according to the height of NBPF, and a further three according to MAPn. The results in all six subgroups were compared.
First, the patients were divided into three groups: Group 1 (NBPF not higher than 10%; non-dippers), Group 2 (NBPF 10–15%, dippers) and Group 3 (NBPF>15%; extreme dippers). There were four patients in Group 3 whose NBPF was greater than 20% (extreme dipper) and for these patients only the mean values of the analyzed parameters have been given since, because of their small number, a separate statistical analysis was not carried out.
Secondly, the patients were also divided into three groups: Group A (MAP<79 mmHg), Group B (MAPn 79–87 mmHg) and Group C (MAPn>87 mmHg); 87 mmHg was the highest value of MAPn, which is recognized as normal according to the recommendations of the ESH, and 79 mmHg is the value 10% smaller than normal (Citation17).
The concordance of the examined parameters with a normal distribution were checked using the Shapiro–Wilk test and, additionally, the D’Agostino–Pearson test. We did not observe any differences between the results of those tests. When we observed statistical significance while doing ANOVA test for normal distribution, the Bonferroni test for multiple comparisons was used. For the parameters with an abnormal distribution, the Kruskal–Wallis with Dunn's multiple comparison test was used.
The results are presented in the form of the arithmetical mean and the standard deviation. Statistical hypotheses were verified at the p < 0.05 level of significance.
The calculations were performed using the Statistica PL 6.0 statistical package and GraphPad Prism 5.01. Graphic illustrations of the calculations were also performed using the Statistica PL 6.0 package.
Results
Arterial BP and IOP
The MAP in the study population, diagnosed using ABPM, was 122.3±11.4/76.84±6.7 mmHg, and the MAPn was 11.36±5.98% lower than the daytime level. On the basis of the ABPM results from this group of 69 patients with glaucoma and treated AH, 30 proved to have a normal circadian profile of pressure (dipper), 35 of them had reduced pressure fall at night (non-dipper), and in four patients an excessive pressure fall in the night (extreme dipper) was diagnosed.
shows the characteristic parameters for glaucoma, which are important in the ophthalmological examination depending on the relative pressure fall at night. In the whole analyzed group of patients, IOP was normal. No significant differences in IOP during the day and night between the subgroups were found. OPP did not differ significantly during the day, but it was significantly lower in Groups 2 and 3 (both p = 0.0009) when compared with patients in Group 1 (non-dippers).
Table I. The results of ophthalmological examination for the whole group of patients and the comparison of parameters analyzed in the ophthalmological examination between groups, depending on the level of blood pressure fall at night.
RNFL, VFD and VEP
The RNFL showed no differences, while VFD were the greatest in those patients with the greatest BP fall at night (Group 3). The defects differed considerably (p < 0.0001) only with reference to the patients with the lowest NBPF (Group 1). Evaluation of the evoked potentials revealed significant differences between Groups 1 and 3 for VEP L (p = 0.0002) and between Groups 1 and 2 for VEP A and VEP L (p = 0.032 both cases).
Doppler imaging measurements of retrobulbar vessels
The parameters of flow in the vessels of the eye and orbit between the groups, depending on the level of nocturnal BP fall are presented in .
Table II. Comparison of analyzed parameters of flow in the retrobulbar vessels between the groups, depending on the level of night blood pressure fall.
The systolic and diastolic flow rates and the RI in the ophthalmic and central retinal arteries differed significantly between the groups. PSV and EDV values were the lowest in the group with the highest NBPF and largest in the dipper-type patients. Inverse relations were observed for RI in the ophthalmic and central retinal arteries. The parameters of optic flow in the posterior ciliary artery did not show differences between the groups.
ABPM
A full analysis of the results of four patients in Group 3, whose average NBPF value exceeded 20% (24.3±1.7%), was not carried out. However, with a MAP of 132.5 ± 7.9/78.5 ± 6.9 mmHg and comparable values of flow parameters in the tested vessels in these patients, compared with the entire Group 3, we found a greater loss in the visual field of almost 25% (62.5 ± 5% vs 49.7 ±13.7% respectively, p = 0.0317), and the amplitude of evoked potentials was about 48% lower (3.75±2.9 µV vs 7.27±5.65 µV, p = 0.111). In these four patients, a lower perfusion pressure and lesser thickness of the nerve fibers by almost 5%, compared with the entire Group 3, were noticed.
presents the results of the ABPM analysis in groups depending on the level of the night fall. MAPd did not differ between groups, but at night it was significantly higher in patients with low NBPF, compared with the other groups.
Table III. Comparison of blood pressure values between the groups set during ambulatory blood pressure monitoring (ABPM), depending on the level of night blood pressure fall.
MAP level
Therefore, further analysis was carried out after dividing the patients according to the value of MAPn. The summary of ophthalmological examination results obtained in Groups A, B and C is shown in . Compared with the groups discussed above, the average IOPs during the day and night was the highest in patients with the lowest MAPn. Similarly, OPP was the most impaired in patients with the lowest MAPn, both during the day and at night. The comparison of RNFL, VFD, VEP, VEP A and VEP L showed that the results were the least favorable in the group with the lowest MAPn. However, these differences were not statistically significant, except for the VEP L.
Table IV. The results of ophthalmological examination within the groups, depending on the level of mean arterial pressure at night (MAPn).
Analysis of the flow in the vessels of the eye and orbit in groups depending on the MAPn level showed that systolic and diastolic flow in the CRA and systolic flow in the OA were significantly impaired in the group with the lowest MAPn compared with other groups. Significant differences were also revealed in OA-EDV and SPCA-PSV between Groups A and C (). There were no differences in the RI in the examined arteries of eyeglobe and orbit.
Table V. Parameters of the flow in the retrobulbar vessels in the three groups, depending on the value of mean arterial pressure at night (MAPn).
Correlations
Correlations between the relevant parameters for glaucoma, the ophthalmic flows in the retrobulbar vessels and the parameters characterizing BP in the ABPM in the whole group of patients are shown in . Clinical and hemodynamic parameters of the eyeglobe and orbit vessels reveal the strongest dependencies on minimum systolic BP, MAP level during the night and the relative NBPF.
Table VI. The Spearman rang order correlations between the parameters of the retrobulbar flow vessels and the values of arterial blood pressure in ambulatory blood pressure monitoring (ABPM) for the whole group.
Correlations between the parameters of flow in the retrobulbar vessels of the analyzed group of patients and RNFL, VFD, OPPd and OPPn are shown in .
Table VII. The Spearman rang order correlations between the parameters of the flow in the retrobulbar vessels and the ocular perfusion pressure (OPP), retinal nerve fiber layer thickness (RNFL) and visual field defect (VFD) values.
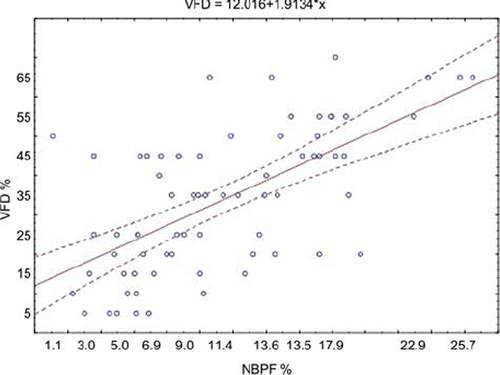
Finally, the correlation between the NBPF and the visual field defect in the whole group of patients is shown on .
Discussion
Increased IOP has long been considered the main risk factor for glaucoma development, and its risk factor control has been the most important element of treatment. It has been pointed out, however, that in some patients, despite effective IOP reduction, progressive glaucoma damage cannot be arrested. This fact suggests that other important risk factors may exist in these patients, factors that are not related to IOP (Citation2,Citation3). There is evidence showing the role of a vascular component in the pathogenesis of glaucoma, including hemodynamic changes (Citation18,Citation19). It has been pointed out in several publications that in patients with glaucoma progression, despite adequate IOP reduction, the flow in the vessels of the retina, choroid and optic disc is slower than in healthy subjects (Citation18,Citation20–22). Some suggest that the perfusion abnormalities in the arteries of the eyeglobe and orbit, which result from BP fluctuations, may be an important pathogenic factor in glaucoma damage and which, regardless of the IOP, cause damage to the optic nerve (Citation18,Citation20–22).
The connection between hypertension and glaucoma progression has been confirmed in several epidemiological studies (Citation23–25). On the basis of these studies, a quotient of POAG development chance in AH, in a range from 1.55 to 2.1 (Citation26) has been defined. The Blue Mountains Eye Study has indicated AH as an independent factor for glaucoma development (Citation26,Citation27). The influence of increased systolic BP on progression of the disease is, however, complex and not fully understood. Hypertension is a chronic disease, which induces changes of a sclerotic nature in small vessels. As a result, peripheral resistance is increased and organs are damaged (Citation28–30). In turn, the Barbados Incidence Study of Eye Diseases I (BISED I) showed an inverse correlation between AH and the risk of POAG occurrence. In this 4-year study, patients with AH had half the risk of developing glaucoma, compared with patients with low systemic pressure, regardless of their age (Citation31). The Barbados Incidence Study of Eye Diseases II (BISED II) also showed a trend to glaucoma development, a trend which declines with an increase in BP (Citation32).
The results of several studies examining the impact of systemic BP on glaucoma occurrence show that hypotension is also a risk factor for glaucomatous changes. These studies even suggest that hypotension is a much more important risk factor than hypertension (Citation16,Citation33–35). Hypotension may lead to reduced perfusion pressure in the vessels of the eyes and may cause changes of a glaucomatous nature (Citation19). In the Thessaloniki Eye Study, it was observed that a decrease in DBP<90 mmHg during AH treatment resulted in changes within the optic disc, even in patients without glaucoma (Citation36).
Our observations also apply to patients with treated hypertension in whom good control of arterial BP was confirmed by measurements taken using a traditional method and ABPM. IOP evaluation in our study population also indicated an effective glaucoma treatment. In several examinations, there was a similar group of patients examined. In some patients, there was a rapid progression of glaucoma and the only explanations for this fact were the nocturnal BP falls (Citation37–42).
We therefore divided our patients into three groups: two “dipper” groups with a nocturnal BP fall of more than 10% and 15% and one “non-dipper” group with a nocturnal BP fall of less than 10%. The reason for this unusual distribution was the fact that only four patients (5.7% of all patients) had nocturnal BP falls of more than 20% and were therefore classified as “extreme dippers”. We were not able to confirm earlier reports that nocturnal BP falls of more than 20% relate to the majority of patients with glaucoma (Citation43).
In the “dipper” subgroups, nocturnal perfusion pressure was lower and the visual field defect was greater than in the “non-dipper” group. By contrast, the thickness of the nerve fibers did not differ significantly with reference to the group of up to 10% nocturnal BP fall. Divergent views have been expressed in the literature concerning the significance of the level of nocturnal BP fall for the progress of glaucoma. Graham et al. (Citation42) demonstrated greater progression in “dippers” compared with “non-dippers”. Furthermore, Tokunaga et al. (Citation44) found a relationship between the progression of visual field defect and the circadian profile, not only of “non-dippers” but also of “extreme dippers” (NBPF>20%).
Our results showed no significant differences in IOP between dippers and non-dippers but we did find a considerable difference in nocturnal perfusion pressure, which was much higher in the “non- dippers” group. The Barbados Eye Study results prove that diastolic perfusion pressure is the most stable vascular risk factor for glaucoma (Citation45). The Baltimore Eye Study showed that low diastolic perfusion pressure is closely associated with POAG, especially when its value is lower than 50 mmHg (Citation16). In another study, the diastolic OPP value, which increases the risk of developing glaucoma, was at the level of <70 mmHg (Citation23) that corresponded to the values that were observed in our study in the “dipper” groups. This may mean that in “dippers” with AH and POAG, excessive lowering of systemic pressure may cause a devastating and unintended effect within the optic nerve. The results of another study (Citation46) have been consistent with this observation, since in patients with impaired self-regulation of ocular circulation and raised IOP, a systemic pressure fall and accompanying reduction in OPP have led to ischemia and damage to the optic nerve.
High values of resistance and reduced flow during systole and diastole in the assessed vessels of the “dipper” group, the factors that were confirmed in our study and also showed by Kaiser et al., can be indirect causes of rapid glaucoma progress (Citation21,Citation47). There are also reports showing that there is a relationship between the flow of blood through the retina and changes in the optic nerve (Citation48–53). In relation to these parameters, we have shown differences between the “dipper” group, with nocturnal BP falls between 10% and 15%, and the extreme dipper group with NBPF of more than 15%, to the detriment of the latter.
It should be emphasized that the daily mean values of systolic and diastolic pressure, which were assessed in ABPM, did not differ significantly within the three groups, while the minimum values of systolic and diastolic BP at night, and the mean arterial BP at night were lower in both “dipper” groups than in the “non-dipper” group.
Therefore, we carried out a similar analysis of the ophthalmic parameters and of the flow in the ocular vessels within Groups A, B and C, which related to the nocturnal MAP, i.e. above the norm of normal pressure in ABPM, below the norm and over 10% below the norm. Here substantial differences not only in vessels perfusion, but also in the level of IOP, were noticed. What is more, we proved that both systolic and diastolic flow in the OA and CRA was smallest in patients with normal MAPn and largest in patients with MAPn increased by over 10%. This is consistent with the results of Gherghel et al. (Citation54), who revealed the influence of a large MAPn fall on the hemodynamic parameters in the vessels of the eye. The flow in these arteries is responsible for, among other things, vascularization of the optic disc. Significantly reduced blood flow in these vessels, especially diastolic flow in patients with low MAPn, can lead to nerve ischemia and degenerative changes. This fact explains the pathomechanism of the phenomenon that has been described earlier by other authors, who have proved that nocturnal hypotension may cause ischemia of the optic nerve (Citation55). Our studies confirm the observations of Grunwald et al. (Citation56), who demonstrated a positive correlation between the flow of blood through the vessels supplying the optic nerve and BP, concluding at the same time that hypotensive treatment leads to a deterioration of the blood flow through the optic nerve. They also showed that the flow is about 29% lower in people with glaucoma, compared with healthy subjects and that, in patients with untreated AH, the flow normalizes. During a 5-year observation, the impact of the amount of pressure fall on visual field deterioration has been confirmed (Citation42). In our study, we also observed a significant relationship between perfusion in the ophthalmic and central retinal arteries, the nerve fiber thickness and the size of the visual field defect.
We did not find any significant differences in nerve fiber thickness or in the field of vision in the groups with different MAP. Furthermore, in the correlation analysis, the amount of flow in the arteries of the eye showed a stronger dependence on the level of nocturnal pressure fall and minimum diastolic BP at night than on the level of mean pressure at night. Moreover, the size of visual field defect showed a correlation with NBPF and minimal diastolic BP at night, and did not show a dependence on MAPn, which is consistent with the results of Galassi et al. (Citation57). These workers proved that, in patients with low diastolic blood flow and a large RI in the OA, there was more progress in the field of vision loss. Our data confirmed reports by other authors that low systemic pressure such as the one observed, for instance, in shock may cause changes in the optic nerve similar to those caused by glaucoma (Citation15,Citation58). In our study, we have proved, for the first time, that there is a relationship between the level of nocturnal BP fall and a reduction in thickness of the nerve fibers.
The hemodynamic mechanism of this phenomenon is complex. Excessive fluctuations in day/night pressure, and not the persistence of low pressure at night, are of greater pathogenic significance. It can therefore be assumed that in patients with hypertension the impairment of fast autoregulation mechanisms of circulation within the eyeball, which results from hypertensive arterial remodeling, plays an important role in the changes in the optic nerve. The impairment manifests itself where there is an excessive BP fall at night and where there are low diastolic pressure values that are obtained periodically.
Our observations indicate the possibility of glaucoma progression in subjects with AH in whom hypotensive therapy has normalized the pressure values and in whom the pressure values fall considerably at night. This requires caution in introducing aggressive hypotensive therapy in patients with AH and glaucoma, or in those who are at risk from these diseases. Our analysis has proved, for the first time, that low nocturnal BP significantly reduces the flow of blood through the vessels of the eyeball, causes degeneration of nerves and, at the same time, correlates with the size of visual field defect. Further multi-centre studies, which will determine safe values of minimal diastolic pressure in patients with hypertension and glaucoma, are required.
Our study has several limitations. It is cross- sectional and therefore we are not able to discuss associations between a marked fall in nocturnal BP and progress of glaucoma. Now, we are continuing to work on a prospective study of this problem and according to our preliminary results, the association is very feasible. In ABPM examination, the hours 08:00–22:00 were considered as period of daily activity and the hours between 22:00 and 08:00 were considered as the period of sleep independently of patients diaries but all of our patients we examined in hospital, in which quiet hours are compulsory after 22:00.
In summary, in patients with glaucoma and well-controlled hypertension, a normal nocturnal BP fall of more than 10% is associated with a greater visual field defect and greater degeneration of the optic nerve fibers, which may be the result of reduced perfusion in the ophthalmic and central retinal arteries in this group of patients. The lowest minimum diastolic pressure and the level of the reduction in the nocturnal BP, but not the absolute value of the average arterial BP at night, should be included in the group of specific risk factors in patients with open-angle glaucoma and hypertension.
The dependencies listed by us show that, in this group of patients, there exists the need for carrying out ABPM with the aim of determining the fall in nocturnal BP. These findings also suggest that excessive lowering of BP at night should be avoided in patients with glaucoma and hypertension.
Acknowledgments
We wish to express our sincere gratitude to Professor Jeffrey Shaw for his assistance in the preparation of the English language version of this manuscript.
Potential conflict of interest
None.
References
- Hulsman CA, Vingerling JR, Hofman A. Blood pressure, arterial stiffness and open-angle glaucoma: The Rotterdam Study. Arch Ophthalmol. 2007;125:805–812.
- Dielemans I, Vingerling JR, Algra D. Primary open-angle glaucoma, intraocular pressure and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology. 1995;102:54–60.
- Salim S, Shields B, Glaucoma and systemic disease. Surv Ophthalmol. 2010;55:64–77.
- Wilson R, Martone J. Epidemiology of chronic open-angle glaucoma. Ritch R, Shields M, Krupin T, . The glaucomas. 2nd St. Louis, MO: Mosby; 1996. 753–768.
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, . The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–713; Discussion 829–830.
- Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European Glaucoma Prevention Study. Ophthalmology 2005;112:366–375.
- Emre M, Sorgul K. Ocular blood flow alteration in glaucoma is related to systemic vascular deregulations. Br J Ophthalmol. 2004;88:662–666.
- Plange N, Kaup M, Daneljan L, Predel HG, Remky A, Arend O. 24-h blood pressure monitoring in normal tension glaucoma: Night-time blood pressure variability. J Human Hypertens. 2006;20:137–142.
- Hayreh SS. Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol. 1999;10:474–82.
- Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96.
- Bresson-Dumont H, Bechtoille A. Arterial hypotension in glaucoma of normal or moderately high pressure. J Fr Ophtalmol. 1995;18:128–134.
- Choi J, Joeng J, Cho H. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: A risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47:831–836.
- Choi J, Joeng J, Cho H. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: A risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47:831–836.
- Mancia G, de Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . Management of Arterial Hypertension of the European Society of Hypertension/European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Drance SM, Sweeney VP, Morgan RW, Feldman F. Studies of factors involved in the production of low tension glaucoma. Arch Ophthalmol. 1973;89:457–465.
- Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure and primary open gluacoma. Arch Ophthalmol. 1995;113:216–221.
- Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness and open angle glaucoma: The Rotterdam Study. Arch Ophthalmol. 2007;125: 805–812.
- Wolf S. Retinal hemodynamics using scanner laser ophthalmoscopy and hemorheology in chronic open angle glaucoma. Ophthalmology. 1993;100:1561–1566.
- Gasser P. Why study vascular factor in glaucoma? Internat. Ophthalmol. 1999;22:221–225.
- Duijm HF, Vandenberg TJ, Greve EL. Choroidal hemodynamics in glaucoma. Br J Ophthalmol. 1997;81:735–742.
- Pruente CH, Flammer J. Choroidal angiography findings in patients with glaucoma-like visual field defects. Perimetry Update 1988/1989. Amstelveen: Kugler and Ghedini; 1989. 325–327.
- Ulrich CH. Stoerung der peripapillaeren Mikrozilkulation bei Glaukomapatienten. Ophthalmologie. 1993;90:45–50.
- Leighton DA, Phillips CI. Systemic blood pressure in open-angle glaucoma, low tension glaucoma and the normal eye. Br J Opththamol. 1972;56:447–453.
- Dielmans I, Vingerling JR, Algra D, Hofman A, Grobee DE, de Jong PT. Primary open-angle glaucoma, intraocular, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology. 1995;102:54–60.
- Bonomi L, Marchini G, Maraffa M, Bernardi P, Morbio P, Varotto A. Vascular risk factors for primary open angle glaucoma, the Egnna Neumarkt Study. Ophthalmology. 2000;107:1287–1293.
- Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: The Blue Mountains Eye Study. J Glaucoma. 2004;13:319–323.
- Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–1460.
- Francois J, Neetens A. The deterioration of the visual field in glaucoma and the blood pressure. Doc Ophthalmol. 1970;28:70–109.
- Gramer E, Tausch M. The risk of profile of the glaucomatous patient. Curr Opin Ophthalmol. 1995;6:78–88.
- Rouhiainen HJ, Terasvirta ME. Hemodynamic variables in progressive and non-progressive low tension glaucoma. Acta Ophthalmol. 1990;68:34–36.
- McLeod SD, West SK, Quigley HA, Fozard JL. A longitudinal study of the relationship between intraocular and blood pressures. Invest Ophthalmol Vis Sci. 1990;30:2361–2366.
- Leske MC, Wu SY, Hennis A, Honaken R, Nemesure B. BESs Study group. Risk factors for incident open-angle glaucoma: Bardabos Eye studies. Ophthalmology. 2008;115:85–93.
- Bayer AU, Keller ON, Ferrari F. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer's disease and Parkinson's disease. Am J Ophthalmol. 2002;133:135–137.
- Wilson MR, Hertzmark E, Walker AM. A case control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987;105:1066–1071.
- Wong T, Mitchell P. The eye study in hypertension. Lancet. 2007;369:425–435.
- Topouzis F, Coleman AL, Harris A. Association of blood pressure status with the optic disc structure in non glaucoma subjects: The Thessaloniki eye study. Am J Ophthalmol. 2006; 142:60–66.
- Pache M, Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Ophthalmology. 1984;91:1690–1694.
- Hayreh SS, Zimmerman MB, Podhajsky P, Aylward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–624.
- Bechetoille A, Bresson-Dumont H. Diurnal and nocturnal blood pressure drops in patients with focal ischemic glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994;232:675–679.
- Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69.
- Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–867.
- Graham SI, Drance SM. Nocturnal hypotension: Role in glaucoma progression. Surv Ophthalmol. 1999;43:10–16.
- Muzyka M, Niżankowska MH, Koziorowska M, Zając-Pytrus H. Występowanie nocnej hipotonii tętniczej u chorych na jaskrę pierwotną otwartego kąta z normalnym ciśnieniem. Klinika Oczna. 1997;99:109–114.
- Tokunaga T, Kashiwagi K, Tsumura T, Taguchi K, Tsukahara S. Association between nocturnal blood pressure reduction and progression of visual field defect in patients with primary open glaucoma or normal tension glaucoma. JPN J Ophthalmol. 2004;48:380–385.
- Leske MC. Incidence of open angle glaucoma: The Barbados Eye Studies. The Barbados Eye Group. Arch Ophthalmol. 2001;119:89–98.
- Deokule S, Weinreb RN. Relationship among systemic blood pressure, intraocular pressure and open angle glaucoma. Can J Ophthalmol. 2008;43:302–307.
- Kaiser HJ, Schoetzau A, Stumpfig D, Flammer J. Blood flow velocities of the extraocular vessels in patients with high-tension and normal tension primary open glaucoma. Am J Ophthalmol. 1997;123:320–327.
- Logan JF, Rankin SJ, Jackson AJ. Retinal blood flow measurements rim damage in glaucoma. Br J Ophthalmol. 2004;88: 1049–1054.
- Plange N. Papillare Fullungsdefekte in Fluoreszein Angiographien bei Glaukom-Eine retrospektive klinische Studie. Klinische Monatsblatter fuer Augenheilkunde. 2001;218: 214–221.
- Galassi F, Nuzzaci G, Sodi A, Casi P, Vielmo A. Color Doppler imaging in evaluation of nerve blood supply in normal and glaucomatous subjects. Int Ophthalmol. 1992;16: 237–276.
- Yamazaki Y, Hayamizu F. Analysis of ophthalmic arterial flow by color Doppler imaging in glaucomatous eyes (in Japanese). Nippon Ganka Gakkai Zasshi. 1994;98:1115–1120.
- Nicolea MT, Walman BE, Buckley AR, Drance SM. Ocular hypertension and primary open-angle glaucoma: A comparative study of their retrobulbar blood flow velocity. J Glaucoma. 1996;5:308–310.
- Matthiessen ET, Zeitz O, Richard G, Klemm M. Reproducibility of blood flow velocity measurements using color decoded Doppler imaging. Eye. 2004;18:400–405.
- Gherghel D, Orgul S, Gugleta K, Flammer J. Retrobulbar blood flow in glaucoma patients with nocturnal over-dipping in systemic blood pressure. Am J Ophthtalmol. 2001;132:641–647.
- Hayreh SS. Progress in the understanding of the vascular etiology of glaucoma. Curr Opin Ophthalmol. 1994;5II:26–35.
- Grunwald JE, Piltz J, Hariprasad SM, Doupont J, Maguire MG. Optic nerve blood flow in glaucoma: Effect of systemic hypertension. Am J Ohthalmol. 1999;127:516–522.
- Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: A color Doppler imaging study. Arch Ophthalmol. 2003;121:1711–1715.
- Hayreh SS. Blood flow in optic nerve head factors that may influence it. Prog Retin Eye Res. 2001;20:595–624.