Abstract
Background. The aim of this study was to obtain epidemiological data and to determine the prevalence of adolescent hypertension implementing a blood pressure (BP) screening. Methods. We performed a cross-sectional, population-based survey in a major Hungarian city (Debrecen, population 230,000). After a 10-min resting period, three consecutive BP measurements were taken. Results. Complete records were obtained for 10,194 subjects (5163 boys and 5031 girls). The mean age was 16.6±1.0 years. BP for boys was higher than for girls (ΔBPsyst=11 mmHg; ΔBPdiast=2 mmHg, p<0.001). A significant decrease was observed in BP during the three consecutive measurements (time 1–3: ΔBPsyst=4 mmHg; ΔBPdiast=2.5 mmHg; p<0.001). Systolic and/or diastolic BP exceeded the age-, gender- and height-adjusted 90th percentile in 1614 (15.84%) adolescents. Following two lots of three extra measurements on 1461 subjects of this sub-sample, hypertension (systolic and/or diastolic BP exceeded the 95th percentile) was confirmed in 2.12% of the subjects (male: 2.27%, female: 1.97%). Considering there were individuals either already diagnosed with hypertension (n=19) or refusing further participation, the prevalence of hypertension was 2.53% in adolescents in the age range 15–18 years. Conclusion. Our population-based study was the first to determine the point-prevalence of adolescent hypertension in Central-Eastern Europe. Identifying and following-up cases of confirmed hypertension is inevitable to prevent or delay the manifestations of target organ damage and reduce hypertension-related mortality.
Introduction
Cardiovascular morbidity usually occurs in adulthood, but essential hypertension may often manifest in adolescence (Citation1). Prevalence of hypertension in adolescence is markedly lower than in adulthood. Literature findings are inconclusive on 15–18-year-old adolescents. Large-scale studies place the range between 0.5% and 12% (Citation2–5). The wide range in prevalence is primarily attributed to the fact that diagnostic criteria did not used to be uniform. There are also marked variations in the results of different population studies conducted according to the latest guidelines (Citation6–8). The most probable prevalence of adolescent hypertension is estimated to be 1–1.5% (Citation9).
Leading medical societies (Citation1,Citation9–10) recommend screening of all children beyond the age of 3 and all adolescents for high blood pressure (BP) on a yearly basis as part of a routine physical examination. A diagnosis of hypertension cannot be established by a single BP measurement. Consecutive and repeated measurements are necessary to obtain reliable results and make precise conclusions (Citation1,Citation9). BP usually decreases during control measurements, whether related to accommodation, reduced stress, “regression to the mean” or a combination of the above. Circumstances and techniques of BP measurements to detect elevated BP in adolescents are also very important. Standard resting conditions, validated equipment and repeated measurements are of high importance (Citation11).
Hypertension is not a qualitative but a quantitative deviation from the normal value. However, it is obligatory to define the abnormal threshold for everyday clinical practice (Citation12). The definition of hypertension in adolescents and in children is largely epidemiological: Hypertension is present if BP exceeds the mean value by two standard deviations (+2SD) (Citation1,Citation9).
Adolescents’ BP values are compared with reference values in nomograms and percentile curves stratified for age, gender and height. The guideline published in 1996 summarized key points of epidemiological studies representing data from 61,000 children (Citation13). Adolescent BP varies significantly, showing geographical and ethnic patterns. Therefore, one should determine the BP distribution of the population in order to identify properly the reference range to which actual BP measurements should be compared. Lack of such population reflective data may risk the over or under diagnosis of hypertension (Citation13).
BP is normal if neither systolic nor diastolic values exceed the respective subgroups’ 90th percentiles. If systolic and/or diastolic BP is higher than the 90th percentiles value of the age-, gender- and height-stratified subgroups, further measurements are needed (Citation1,Citation9). BP is called high-normal if the systolic and/or diastolic value is between 90th and 95th percentiles. Based on the current guideline (Citation1), hypertension is diagnosed if the average of at least three consecutive measurements taken on three different occasions exceeds the 95th percentile value of the age-, gender- and height-specific subgroup's BP. Should systolic and diastolic BP values belong to different groups (normal BP, high-normal BP, hypertension), the adolescent then has to be designated a more serious case (Citation1,Citation9).
We are unaware of any representative, popultion-based hypertension study in 15–18-year-old adolescents either in Hungary or in Central-Eastern Europe to date. Therefore, the current study addresses the following aims:
to identify the point prevalence of adolescent hypertension in Debrecen;
to obtain epidemiological data of adolescents;
to compare the results of three consecutive BP readings;
to perform a BP screening program on Debrecen's secondary school students (15–18 years) according to international guidelines.
Subjects and methods
The study was performed in a cross-sectional, population-based setting. The subjects attended high school during the study period. Each student in all 26 municipal high schools had the potential to become a participant except for those who were absent on the day that the measurements were taken.
Blood pressure measurements were obtained from every participant three consecutive times. Weight and height were recorded as well. The study was carried out with the written consent of the Ethics Review Board of the University of Debrecen.
General methods and procedures (pilot study, training of the investigators, responding to questionnaires, technique of BP measurement, etc.) have been published in detail elsewhere (Citation14). Here we will only discuss the key elements of the measurement procedures and data collection.
Blood pressure measurements
All measurements were taken in classrooms between 08:00 and 13:00 h under standard circumstances. After 10 min of rest, systolic and diastolic blood pressures were measured in the sitting position on the right arm using validated OMRON M4 digital oscillometric manometer (OMRON Healthcare GmbH, Hamburg, Germany) (Citation15,Citation16). If the forearm circumference was 34 cm or over, an obese cuff was used. Three measurements were performed in each case with 5-min intervals and the average values of the systolic and diastolic blood pressure were used for further analysis.
Subgroup divisions, interpreting results
We applied the criteria described by The Task Force on Blood Pressure in Children (Citation9,Citation13). Based on age, gender and height, subjects were divided into 32 subgroups. In terms of age, we formed the following four subgroups: 15-year-olds (14.50–15.49) 16-year-olds (15.50–16.49), 17-year-olds (16.50–17.49) and 18-year-olds (17.50–19). Taking gender into account (male, female), eight subgroups were created. Following the guidelines (Citation13), we classified additional subgroups by subjects’ height quartiles (<25th percentile, 25–49th percentile, 50–74th percentile and ≥75th percentile) in each group. Then we calculated BP nomograms of all 32 subgroups, especially for the 90th and 95th percentile values.
Further measurements in case of elevated blood pressure
Based on guidelines (Citation1,Citation9), repeated measurements (three consecutive measurements on two further occasions) are needed in adolescents whose systolic and/or diastolic BP exceed the 90th percentile value for age-, gender- and height-specific subgroups. Thus, 3×3=9 BP measurements were performed in this group in order to prove subjects’ hypertension. The selection of hypertensive adolescents and the assessment of hypertension prevalence were based on the average of the nine BP measurements, with the consideration of considering the initial 95th percentile value of BP measurements obtained for the entire population.
Data coding and statistical analyses
Data were recorded with Access for Windows using a “cross-check” technique. Statistical analyses were performed using SPSS Windows version 11.0. Descriptive statistics included the mean, standard deviation and proportions (%). We assessed normal distribution of sample characteristics by visual aids (histograms) as well as with the Kolgomorov–Smirnow test.
To indicate differences between groups, independent sample t-tests and repeated-measures analysis of variance (rmANOVA) were used. Level of significance was set at 5%.
Results
The final sample consisted of 10,359 adolescents, after 22 had refused participation. We also recorded weight and height of a total of 10,213 individuals (98.59%). At the time of the investigation, 19 individuals (0.2%) had already been treated for hypertension. Their data were considered only for determining the prevalence of the disease. In subsequent analyses, we considered data only from 10,194 individuals. Demographic characteristics of subjects are summarized in .
Table I. Sample characteristics.
Blood pressure values (both systolic and diastolic) decreased during the three consecutive measurements in the entire study population as well as in gender-specific subgroups. A significant difference was found both between genders (boys vs girls) and within subjects (at the three time points) in each analysis. Boys had significantly higher systolic and diastolic BP (ΔBPsyst=11 mmHg; ΔBPdiast=2 mmHg, p<0.001) relative to girls. The mean difference, tested by rmANOVA, was significant both for systolic (F=413.51; p<0.001) and diastolic (F=180.98; p<0.001) values. There was a difference of 4 mmHg in systolic and 2.5 mmHg in diastolic BP between the first and the third measurement ().
Table II. Differences in blood pressure (BP) by repeated measurements.
As shown in , having completed the three BP measurements we excluded hypertension in 8580 (84.16%) subjects, whose systolic and diastolic BP was less than the age-, gender- and height-adjusted 90th percentile defined by the authors (Citation14). Age-, gender- and height-adjusted 90th percentile systolic and/or diastolic BP values were approximated or exceeded by 1614 (15.84%) adolescents, thereby making it necessary to perform three extra measurements on two further occasions. We repeated measurements for another 1461 subjects, while 153 individuals were excluded from further evaluations. After the nine (3×3) measurements were averaged, hypertension was not confirmed in 1245 individuals, since neither their systolic nor diastolic BP average reached the 95th percentile of their respective subgroup. Systolic and/or diastolic BP was higher than the 95th percentile for the age-, gender- and height-specific subgroups in 216 (2.12% of the total and 14.79% of the 1461 subjects suspicious for hypertension for the initial measurement) cases and these were confirmed as hypertension. Of this group, 117 of adolescents were boys and 99 were girls.
Figure 1. Flow-chart of the Debrecen Hypertension Study. Determining the prevalence of adolescent hypertension.
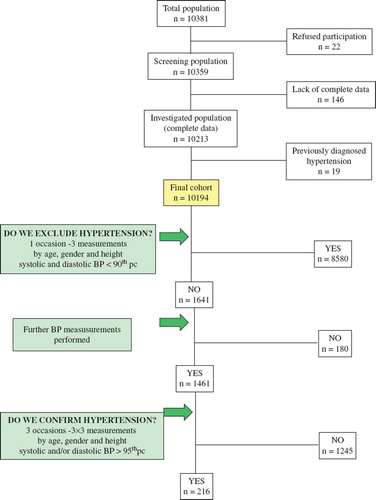
To determine the prevalence of hypertension, two additional factors were also considered. First, control measurements were performed only for 1461 adolescents (90.52%) and not for 1614. Assuming the same prevalence of hypertension, the 153 adolescents excluded earlier from control measurements could have hidden 23 hypertensive cases. Second, 19 adolescents treated for hypertension had already been excluded at the outset of our study. Altogether, 258 (216+23+19) adolescents out of the examined 10,213 had hypertension. Thus, the prevalence of the disease in our population was 2.53% (2530/100,000).
Discussion
The importance of adolescent BP measurements and the derived conclusions are determined by the relationship between adolescent and adult BP. The correlation coefficient (r) of adolescent BP measured repeatedly one year later was 0.62 for systolic and 0.40 for diastolic blood pressure (Citation17). As established by other investigators, the predictive value of the initial BP was 50%, for the BP measured 4 years later. Yet, repeated measurements, long follow-up and standardization have been found to increase results further (Citation18). In the course of a follow-up study of the blood pressure of adolescents belonging to the upper quartile, the BP of 70% remained in the same subgroup, even after 9 years. (Citation19). The relation between adolescent and adult BP has been demonstrated through the “tracking” phenomenon: Children and adolescents whose BP is initially in the upper percentiles will remain in the same range during their growth and development (Citation20,Citation21). Adolescent BP therefore may pose a greater cardiovascular risk in later stages of life. Screening of adolescents is consequently of high importance because adolescent BP is predictive of adult blood pressure.
The current diagnostic recommendation available for practice has been developed on the basis of BP nomograms from 10 epidemiological surveys conducted between 1976 and 1991 (Citation9,Citation13). All studies were done in the USA, and almost half of the participants were non-Caucasians. Nomograms were calculated using single BP measurements because some studies measured BP only once. Because of wide geographical and ethnic variations, normal BP values of adolescents 15–20 years ago were representative of past populations but may not be applicable to the Hungarian adolescents of today (Citation14). No previous population-based, representative study has been performed to examine the BP and the prevalence of hypertension in 15–18-year-old adolescents in Hungary or in Central-Eastern Europe.
Several earlier epidemiological studies assessed BP only once for each subject. In recent years, obtaining separate readings three times apart has gained more ground; actual pressure is calculated as the average of these values. Reports confirm that while systolic pressure decreases with the increased number of measurements (2.5 and 3.5 mmHg on average), change in diastolic BP is negligible (Citation22,Citation23). The difference in systolic BP (4 mmHg) in our sample was significant. However, contrary to Burke and his colleagues’ conclusions, our findings showed a significant 2.5-mmHg diastolic drop.
Diagnosing hypertension, especially in adolescents, is a task of great responsibility because our decision may initiate treatment for decades to come. For a more refined diagnosis, our study has demonstrated the necessity of repeated measurements. Blood pressure measured once (even if three times apart) will only help identify truly normotensive cases and distinguish them from those requiring further follow-up. Rather, hypertension should be confirmed when the average of three different measurements (3×3) exceeds the age-, gender- and height-adjusted 95th percentile threshold of the subgroup under study.
Taking three BP measurements is also advised for everyday practice. To designate the adolescent correctly, one measurement would be insufficient as shown in our research. Decisions based on single readings can yield false results and lead to over-diagnosing hypertension, since higher values have been observed at first measurements.
There is also considerable variation in the results of studies on the prevalence of adolescent (15–18 years old) hypertension; large-scale studies report a range between 0.5% and 12%. This wide range in prevalence is primarily attributed to the fact that planning, arrangement and diagnostic criteria are different. Recently accepted guidelines and studies following these recommendations place actual prevalence between 1% and 1.5% (Citation9,Citation13). However, remarkable differences may be observed across populations of distant geographical areas, which may be related to variations in ethnicity and genetic makeup.
In our sample, the prevalence of 2.53% exceeded expected results based on international epidemiological studies. It has been documented that the more often blood pressure is measured the more hypertensive adolescents are discovered. To illustrate geographical and ethnic differences, let us recall that the prevalence of adolescent hypertension is 0.5% in India (Citation24), 1.1% in the USA (Citation2) and 2.2% in Israel (Citation25). In contrast, data from Southern Europe show higher frequencies; adolescent hypertension is estimated at 3% in Greece (Citation3) and 4% in Turkey (Citation26). Poland, geographically close to Hungary, reported the highest prevalence among the Central-Eastern European countries (7%) (Citation4). Hypertension of the youth varies further to the north of Europe; the prevalence in Estonia was between 6% and 12% (Citation5). Such variations have the potential to impose certain restrictions on the accuracy of guidelines when applied to populations from different locations.
Old military data in the USA (Citation27) showed the transient rise in blood pressure and heart rate predicts future hypertension and numerous studies of adolescents with increased BP at one occasion have described quite extensive pathophysiological changes associated with the high BP (Citation28). Though the prevalence of adolescent hypertension was 2.53%, much larger groups (n=1614, 15.83%) may in fact be at risk.
Results from the present research can be extended, with certain limitations, to the other regions of Hungary. Despite limitations, we believe that our data constitute a valid reference for purposes of evaluation and comparison of adolescent hypertension beyond current geographical boundaries. This study implemented the first population-based hypertension screening in Central-Eastern Europe, involving more than 10,000 adolescents in the age range 15–18 years. During this survey process, we identified adolescents with occasionally elevated BP, and performed further measurements. It was these repeated measurements that revealed the point prevalence of adolescent hypertension.
We followed both epidemiological and prevention goals in the design and implementation of the study. From the epidemiological point of view, we aimed at collecting comprehensive data on attributes of adolescent hypertension. On the other hand, for our study, we emphasized preventive goals, too. The identification of adolescent hypertension as well as the clarification of its origin, treatment and follow-up is of immense importance because they are proven precursors of cardiovascular risks in later life. By accurate and early diagnosis of adolescent hypertension, we may prevent or significantly delay manifestations, complications and target organ damage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, . Management of high blood pressure in children and adolescents: Recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742.
- Sinaiko AR, Gomez-Marin O, Prineas RJ. Prevalence of “significant” hypertension in junior high school-aged children: The Children and Adolescent Blood Pressure Program. J Pediatr. 1989;114:664–669.
- Elisaf M, Papanikolaou N, Letzaris G, Dimoliatis J, Siamopoulos KC. Atherosclerotic risk factors in female students of northwestern Greece. J Hum Hypertens. 1993;7:533–537.
- Mareczek S, Wyka S, Odrobina S, Cebulak B, Ejma-Multański J, Fedak M, . Arterial blood pressure of high school adolescents in Cracow – Screening test. Przegl Lek. 1995;52: 115–118.
- Grünberg H, Thetloff M. The cardiovascular risk factor profile of Estonian school children. Acta Paediatr. 1998;87: 37–42.
- Harrabi I, Belarbia A, Gaha R, Essoussi AS, Ghannem H. Epidemiology of hypertension among a population of school children in Sousse, Tunisia. Can J Cardiol. 2006;1;22: 212–216.
- Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;22; 298:874–879.
- Lande MB, Pearson TA, Vermilion RP, Auinger P, Fernandez ID. Elevated blood pressure, race/ethnicity, and C-reactive protein levels in children and adolescents. Pediatrics. 2008;122:1252–1257.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576.
- Committee on Practice and Ambulatory Medicine. Recommendations for preventive pediatric health care. Pediatrics. 1995;96:373–374.
- Gillman MW, Cook NR, Rosner B, Evans DA, Keough ME, Taylor JO, . Assessing the validity of childhood blood pressure screening: Unbiased estimates of sensitivity, specificity, and predictive values. Epidemiology. 1992;3:40–46.
- Goonasekera CD, Dillon MJ. Measurement and interpretation of blood pressure. Arch Dis Child. 2000;82:261–265.
- National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Update on the. 1987 Task Force Report on High Blood Pressure in Children and Adolescents. Pediatrics. 1996;98: 649–658.
- Pall D, Katona E, Fulesdi B, Zrínyi M, Zatik J, Bereczki D, . Blood pressure distribution in a Hungarian adolescent population: Comparison with normal values in the USA. J Hypertens. 2003;21:41–47.
- Stergiou GS, Yiannes NG, Rarra VC. Validation of the Omron 705 IT oscillometric device for home blood pressure measurement in children and adolescents: The Arsakion School Study. Blood Press Monit. 2006;11:229–234.
- Coleman A, Freeman P, Steel S, Shennan A. Validation of the Omron 705IT (HEM–759-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2006;11:27–32.
- Adams-Campbell LL, Ukoli FA, Silverman JA, Omene JA, Nwankwo MU, Kuller LH. Tracking of blood pressure and anthropometric measures in Nigerian children. J Hum Hypertens. 1992;6:47–51.
- Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;15; 92:1049–1057.
- Sánchez-Bayle M, Muñoz-Fernández MT, González-Requejo A. A longitudinal study of blood pressure in Spanish schoolchildren. Working Group of Cardiovascular Risk Factors in Childhood and Adolescence. Arch Dis Child. 1999;81: 169–171.
- Israeli E, Korzets Z, Tekes-Manova D, Tirosh A, Schochat T, Bernheim J, . Blood-pressure categories in adolescence predict development of hypertension in accordance with the European guidelines. Am J Hypertens. 2007;20: 705–709.
- Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180.
- Burke GL, Webber LS, Shear CL, Zinkgraf SA, Smoak CG, Berenson GS. Sources of error in measurement of children's blood pressure in a large epidemiologic study: Bogalusa Heart Study. J Chronic Dis. 1987;40:83–89.
- Jamieson MJ, Webster J, Philips S, Jeffers TA, Scott AK, Robb OJ, . The measurement of blood pressure: Sitting or supine, once or twice? J Hypertens. 1990;8:635–640.
- Anand NK, Tandon L. Prevalence of hypertension in schoolgoing children. Indian Pediatr. 1996;33:377–381.
- Jaber L, Eisenstein B, Shohat M. Blood pressure measurements in Israeli Arab children and adolescents. Isr Med Assoc J. 2000;2:118–121.
- Uçar B, Kiliç Z, Colak O, Oner S, Kalyoncu C. Coronary risk factors in Turkish schoolchildren: Randomized cross-sectional study. Pediatr Int. 2000;42:259–267.
- Levy RL, White PD. Transient tachycardia;prognostic significance alone and in association with transient hypertension. Med Press Egypt. 1946;38:207–212.
- Fossum E, Høieggen A, Reims HM, Moan A, Rostrup M, Eide I, Kjeldsen SE. High screening blood pressure is related to sympathetic nervous system activity and insulin resistance in healthy young men. Blood Press. 2004;13:89–94.