Abstract
European guidelines recommend a combination of at least two antihypertensive drugs to achieve blood pressure (BP) goals in the majority of patients. In addition, they encourage simplification of treatment regimens using single-pill, fixed-dose combinations (FDCs) to aid compliance. Of the preferred combinations, those based on angiotensin II receptor blockers (ARBs) may be more desirable than those based on angiotensin-converting enzyme inhibitors, because of equivalent efficacy and superior tolerability. Significantly better BP reductions and control rates have been observed with the dual combinations of ARBs with amlodipine or hydrochlorothiazide (HCZT) compared with component monotherapies. Furthermore, in the 15−20% of patients who require triple combination therapy to achieve BP goals, fixed-dose triple combinations with an ARB, calcium-channel blocker and diuretic, which have recently become available, provide significantly better BP reductions and control compared with dual combinations. Within the ARB class, olmesartan stands out as being one that has been recently investigated in a considerable number of studies that are relevant to the modern concept of FDC therapy in terms of both dual and triple combination therapy. The availability of such single-pill FDCs has the potential to deliver strong antihypertensive efficacy with good tolerability and improved compliance.
Introduction
Guidance for European physicians on the management of patients with arterial hypertension has been provided by the European Society of Hypertension (ESH) and European Society of Cardiology (ESC) since 2003, with an update in 2007 (Citation1,Citation2). However, after the publication of the 2007 guidelines, data from several important clinical studies provided new insights into critical aspects of patient management, including blood pressure (BP) goals and the benefits of antihypertensive treatment. As such, a reappraisal of the ESH-ESC guidelines was performed in 2009 by an ESH Task Force (Citation3). Important points made by the reappraisal included reaffirming the validity of the systolic BP (SBP)/diastolic BP (DBP) treatment target of less than 140/90 mmHg for all patients with hypertension. The initiation of antihypertensive therapy in patients with grade 1 hypertension (BP 140–159/90–99 mmHg) who are at low-to-moderate cardiovascular (CV) risk, after lifestyle changes have been tried for a suitable period, was also confirmed.
The reappraisal also questioned the BP target of <130/80 mmHg for patients with diabetes because of the lack of evidence of any clinical benefit associated with trying to reach this lower BP target (Citation3,Citation4). Recent data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and the International Verapamil SR-Trandolapril (INVEST) studies are in line with this, and indicate that excessive reduction of BP levels in hypertensive diabetic patients may even increase the risk of mortality (Citation5,Citation6). Furthermore, the reappraisal stressed the increasing evidence that the combination of at least two antihypertensive drugs is required to achieve BP goals in the majority of patients.
This review focuses on the use of combination therapy in the treatment of hypertension, in particular on the use of treatments based upon blocking the renin–angiotensin system (RAS), and recent developments in fixed-dose combinations (FDCs).
The rationale for combining drugs from different classes
The primary goal of treating hypertension is to attain the maximum possible reduction in the long-term total risk of CV morbidity and mortality, which is achieved by treating elevated BP and reversible risk factors (Citation1,Citation2). The importance of achieving a SBP and DBP <140/90 mmHg was stressed in the 2003 European guidelines (Citation1–3). Yet despite this guidance and the well-established link between hypertension and adverse CV outcomes, BP control rates have continued to remain suboptimal. This was illustrated for example by a cross-sectional analysis of data from the CardioMonitor 2004 survey, which revealed that the proportion of hypertensive patients achieving BP control ranged from 36–46% in five European countries (France, Germany, Italy, Spain and the UK) (Citation7).
One factor that may play a role in the suboptimal rate of BP control seen in many countries is the frequent use of monotherapy. Over the last 20–30 years, the management of hypertension has focused on sequential monotherapy, an approach which starts with a low dose of one agent and either increases the dose or switches to a different agent in the absence of a response or the presence of undesirable side-effects. However, clinical data shows that monotherapy generally lacks sufficient efficacy and the majority of patients require combination therapy with at least two agents to achieve target BP () (Citation1,Citation2,Citation8–10). The 2007 guidelines recommend that low-dose combination therapy be used as a first-line treatment in patients with marked BP elevation and high/very high CV risk and as a second-line treatment in patients with mild BP elevation and low/moderate CV risk (Citation1). The frequent need for the use of combination therapy to achieve BP control, particularly in patients with high CV risk, was reaffirmed in the 2009 reappraisal (Citation3).
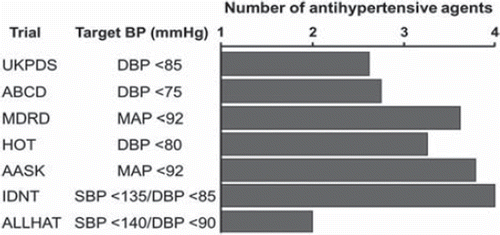
Figure 1. Clinical trials show that the majority of patients require two or more antihypertensive drugs to achieve BP goal (Citation8–10). AASK, African American Study of Kidney Disease and Hypertension; ABCD, Appropriate Blood Pressure Control in Diabetes; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; DBP, diastolic blood pressure; HOT, Hypertension Optimal Treatment; IDNT, Irbesartan in Diabetic Nephropathy Trial; MAP, mean arterial pressure; MDRD, Modification of Diet in Renal Disease; SBP systolic blood pressure; UKPDS, United Kingdom Prospective Diabetes Study.
The benefits of using combination therapy to reduce BP are well established. It has been clearly demonstrated that combining two drugs from different classes with complementary mechanisms of action provides greater BP-lowering efficacy, compared with increasing the dose of monotherapy () (Citation11). The additional BP-lowering efficacy of combination therapy has been estimated to be approximately five times greater than that obtained by doubling the dose of monotherapy, and has also been shown to be paralleled by reductions in the risk of cardiovascular events (Citation11,Citation12).
Compliance
In addition to increasing antihypertensive efficacy, combination therapy offers a further important benefit in terms of favouring compliance (Citation13–15). Despite the recognized benefits of antihypertensive treatment, it is estimated that between 30% and 50% of patients do not comply with antihypertensive therapy (Citation16). Various factors are believed to play a role in poor compliance with antihypertensive medication. These include the chronic and generally symptom-free nature of hypertension, side-effects associated with certain treatments, pill burden and the complexity of some treatment regimens (Citation13,Citation14,Citation16,Citation17). There is a well-established inverse relationship between compliance and pill burden; compliance decreases as the number of daily doses increases (Citation17). Simplifying treatment regimens by decreasing the number of daily doses has been shown to improve compliance (Citation15). The availability of treatments as a single-pill FDC is a straightforward way to simplify treatment, reduce pill burden and encourage compliance.
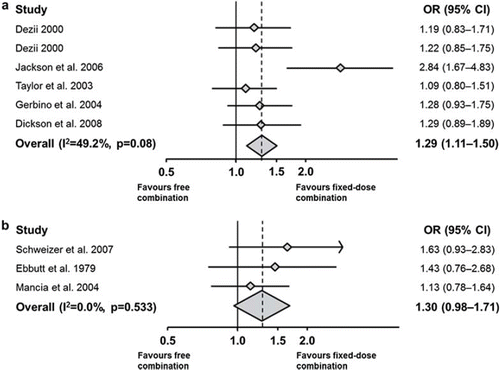
The beneficial impact of administering antihypertensive treatments as FDCs on compliance and BP control, compared with administering the individual drug components separately, was investigated in a recent systematic literature review and meta-analysis (Citation18). Analysis of data from six retrospective studies demonstrated a 29% increase in compliance with the use of an FDC compared with the same drugs given separately as a free-drug combination (odd ratio, OR = 1.29; 95% confidence interval, CI, 1.11—1.50; p = 0.02) (). The use of an FDC also favoured an improvement in BP control; a 30% increase in BP control was observed with an FDC compared with the corresponding free-drug combination (OR = 1.30; 95% CI 0.98–1.71; p = 0.07) ().
Figure 2. The combination of two drugs is more effective than high-dose monotherapy (Citation11). ACE, angiotensin-converting enzyme; SBP, systolic blood pressure.
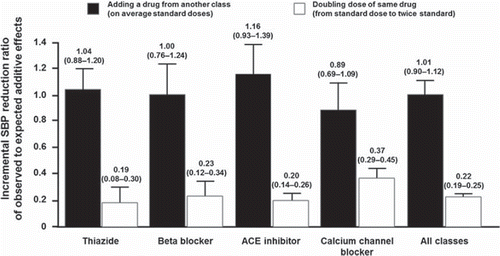
Figure 3. Improved compliance (a) and favoured BP control (b) with fixed-dose antihypertensive treatment, relative to the same therapies administered concomitantly as single formulations (Citation18). CI, confidence interval; OR, odds ratio.
As a method for simplifying treatment and improving compliance, the combining of antihypertensive agents in a single tablet is supported by the 2007 ESH-ESC guidelines (Citation1). Moreover, this recommendation was confirmed by the recent reappraisal of the treatment guidelines, which stated, “Use of fixed dose combinations of two drugs can directly follow initial monotherapy when addition of a second drug is required to control BP, or be the first treatment step when a high cardiovascular risk makes early BP control desirable” (Citation3). The increasing availability of antihypertensive treatments in FDCs is a positive development that should facilitate the uptake of such treatments and help to improve BP control rates.
Triple combination therapy
Despite the increased efficacy provided by combining agents from two different classes, 15–20% of patients do not achieve BP goal even with two agents and require combination therapy with at least three agents () (Citation3). For patients who require a triple combination, the guidelines reappraisal recommends the use of a RAS blocker in combination with a calcium-channel blocker (CCB) and a thiazide diuretic (Citation3).
Fixed dose triple-combinations containing an angiotensin II receptor blocker (ARB), a CCB and a diuretic have recently become available, and clinical studies have demonstrated that triple combination therapy produces significant reductions in BP and increases in BP control compared with dual combinations (Citation19,Citation20).
Combining different antihypertensive agents
Preferred combinations
The preferred combinations listed in the guidelines and reappraisal include, ARBs and thiazide diuretics, angiotensin converting enzyme inhibitors (ACEIs) and thiazide diuretics, ARBs and CCBs, or ACEIs and CCBs (Citation1,Citation3). Outcomes data from large, randomized, clinical trials suggest that some of these combinations, in particular those based on blockers of the RAS, may be more useful than others in terms of reducing cardiovascular morbidity and mortality. The results from the Losartan Intervention For Endpoint reduction (LIFE) study suggest that the combination of an ARB and a thiazide diuretic may be superior to that of a beta-blocker/thiazide diuretic combination (Citation21). An ACEI/CCB combination may be superior to a beta-blocker/thiazide diuretic combination, as demonstrated by the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) (Citation22).
Basing combination therapy upon RAS blockade
The rationale for using a RAS blocker in combination with a CCB or thiazide to lower BP and reduce cardiovascular risk is supported by evidence from a number of trials. Use of an ACEI in combination with a thiazide diuretic is supported by the perindopril protection against recurrent stroke study (PROGRESS), Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) trial and HYVET (Citation23–25). The use of an ARB in combination with a thiazide diuretic is supported by the LIFE and Valsartan Antihypertensive Long-term Use Evaluation (VALUE) studies (Citation21,Citation26).
The ASCOT-BPLA, INVEST and Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) studies are supportive of the use of ACEI/CCB combinations (Citation22,Citation27,Citation28). Additionally, it was demonstrated in the ACCOMPLISH study that benazepril, an ACEI, in combination with a CCB (amlodipine) was more effective than an ACEI/thiazide diuretic (hydrochlorothiazide; HCTZ) combination in reducing CV events in patients who had a high level of CV risk and underlying metabolic complications (Citation28). The results of ACCOMPLISH indicate that in patients with metabolic abnormalities such as impaired glucose tolerance, metabolic syndrome or diabetes, a RAS-blocker/CCB combination may be especially suitable.
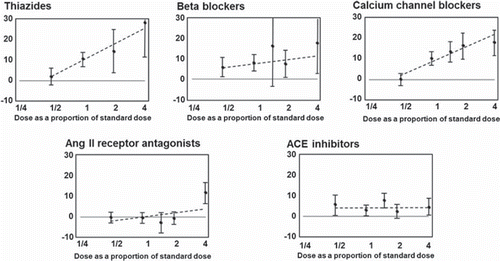
In terms of tolerability, adverse events with CCBs, thiazides and beta-blockers are dose-related, whilst increasing the dosages of ACEIs and ARBs appears to have a less marked effect on adverse events (Citation13,Citation29). Therefore, the use of ARB- or ACEI-based combinations is logical because these agents can be used at higher doses in combination therapy without increasing treatment-related adverse events () (Citation29).
Even though ARBs and ACEIs show excellent tolerability profiles, differences between these two classes do exist. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global End-point Trial (ONTARGET), the ARB telmisartan demonstrated equivalent efficacy to the ACEI ramipril in patients at high risk of vascular events (Citation30). Mean BP reductions in the telmisartan and ramipril groups were similar (7.4/5.0 and 6.3/4.3 mmHg, respectively), and telmisartan was not inferior to ramipril for the primary composite outcome of death from CV causes, myocardial infarction, stroke or hospitalization for heart failure (relative risk 16.7% vs 16.5%, respectively). However, rates of angioedema (0.1% vs 0.3%), hypotensive symptoms (1.7% vs 2.7%) and cough (1.1 vs 4.2) were significantly lower in ARB recipients compared with those who received the ACEI (p < 0.01).
Figure 4. Proportion of patients reporting one or more treatment-related adverse events, according to drug category as a proportion of a standard dose (Citation29). ACE, angiotensin-converting enzyme.
Although angioedema associated with ACEI treatment is rare, it can be potentially serious. The rate of angioedema was 0.2% in a large-scale observational study of more than 195,000 US veterans who initiated ACE therapy (Citation31). A total of 55% of cases occurred within 90 days of treatment initiation, and the increased risk of angioedema extended to beyond 1 year. The authors have projected that approximately one in 2600 new users of an ACEI experiences angioedema within 30 days. Thus, ARB-based combinations may therefore be more desirable than those based on an ACEI because of their equivalent efficacy and superior tolerability. Most of the members of the ARB class have now been combined with the diuretic HCTZ as an FDC, and a few have also been combined with the CCB amlodipine into an FDC (Citation32–34).
Combination therapy based upon ARBs
ARBs block the activity of the RAS at the level of the angiotensin II type 1 (AT1) receptor, and their efficacy depends upon their ability to inactivate the AT1 receptor (Citation35). Pharmacodynamic studies show that differences exist between ARBs in their ability to block the AT1 receptor, and that these differences are generally explained by dosing (Citation35,Citation36). Irbesartan 150 mg was found to be significantly more effective than losartan 50 mg or valsartan 80 mg at blocking the BP pressor response over 24 h to exogenous angiotensin II (p < 0.01 at 4 h; p < 0.05 at 24 h) in normotensive subjects (Citation37). More recently, the BP response to exogenous angiotensin II was shown to be completely blocked by irbesartan 300 mg and olmesartan medoxomil 20 mg and to a lesser extent by valsartan 160 mg and losartan 100 mg. Importantly, the same study also showed that combining an ARB with HCTZ has no effect on the ability of ARBs to block the AT1 receptor (Citation36).
These differences in ability to block the AT1 receptor may have consequences for antihypertensive efficacy in terms of the capacity of ARBs to reduce BP over 24 h. This is supported by the findings of an independent analysis of studies, which used ambulatory BP monitoring (ABPM) to measure 24-h BP control with ARBs (Citation38). The analysis found that the magnitude of the reduction in ambulatory SBP was dependent upon the drug used, and that dose affected the duration of the antihypertensive activity for both systolic and diastolic BP.
One ARB shown in pharmacodynamics studies to produce a strong level of AT1 receptor blockade in relation to dose was olmesartan (Citation36,Citation39,Citation40). This effective blockade of the AT1 receptor may underlie the effective reductions in ambulatory BP seen with olmesartan in the independent analysis, as well as the significantly greater reductions in ambulatory BP over 24 h, the daytime and night-time seen in direct comparisons with several other ARBs (Citation38,Citation41,Citation42).
Multidrug therapy, particularly in higher-risk patients, is a concept supported the ESH-ESC guidelines and by the more recent reappraisal published by the ESH (Citation3,Citation43). Within the ARB class, olmesartan and valsartan are available as a dual combination with HCTZ or amlodipine, and have recently been licensed as triple combinations together with both of these agents and the efficacy and tolerability of these combinations have been studied in various clinical investigations. Olmesartan stands out as being one that has been recently investigated in a considerable number of studies that are relevant to the modern concept of FDC therapy in terms of both dual and triple combination therapy.
Combination therapy with olmesartan
The increased efficacy produced by combining olmesartan with HCTZ or amlodipine, have been demonstrated in numerous clinical studies (Citation44–49). A randomized, double-blind, factorial study in patients with mild-to-severe hypertension demonstrated that combining olmesartan (10, 20 or 40 mg) with HCTZ (12.5 or 25 mg) significantly reduced SBP and DBP and improved BP goal rates compared with the administration of either agent alone (Citation50,Citation51). Furthermore, in patients with moderate-to-severe hypertension who had not achieved an adequate level of BP control with the high dose (40 mg) of olmesartan, the addition of HCTZ produced significant dose-related increases in seated and ambulatory BP, and improvements in seated BP control rates. Adverse events rates and discontinuations with olmesartan 40 mg plus HCTZ 12.5 and 25 mg were similar to those in patients who received olmesartan 40 mg (Citation52).
The combination of olmesartan (10, 20 or 40 mg) with amlodipine (5 or 10 mg) was investigated in patients with mild-to-severe hypertension in a large randomized, double-blind, factorial study. Dose-dependent reductions in seated DBP and SBP were observed with olmesartan in combination with amlodipine that were significantly greater than each of the component monotherapies, and more patients achieved BP goal with olmesartan plus amlodipine. Combination treatment was well tolerated, the incidence of adverse events, including those that led to discontinuation, was comparable across the treatment groups, and the combination of olmesartan 40 mg with amlodipine 10 mg reduced the rate of peripheral oedema associated with high dose amlodipine (Citation48). A further study in patients with moderate-to-severe hypertension who showed insufficient BP control on amlodipine 5 mg, found that adding olmesartan (10–40 mg) produced significant reductions in seated and ambulatory BP, and increases in BP goal rates compared with patients randomized to continue amlodipine 5 mg (Citation46,Citation49).
Clinical studies using stepwise treat-to-target algorithms based upon olmesartan plus HCTZ have shown that such an approach can produce good levels of goal rate achievement in a range of patients including the elderly, and those with diabetes, or more severe hypertension (Citation45,Citation53,Citation54). Similar findings have been obtained in stepwise treat-to-target studies that involved the addition of amlodipine to olmesartan (Citation55,Citation56). Across these studies, BP reductions and BP goal rates were dose dependent and greatest when olmesartan 40 mg was combined with the high dose of HCTZ (25 mg) or amlodipine (10 mg). Of note here is the recent ROADMAP study, which involved approximately 4400 patients with type 2 diabetes and at least one other CV risk factor. In ROADMAP, patients were treated with olmesartan 40 mg or placebo plus additional antihypertensive agents (except ACEIs or ARBs) as needed to control BP. An analysis of the antihypertensive treatments used has not yet been made available, but the nature of the patient cohort means it is likely that the majority of patients were receiving combination therapy. This is of interest because BP is generally hard to control in diabetic patients with hypertension, but in ROADMAP nearly 80% of olmesartanrecipients achieved the BP goal <130/80 mmHg, and many patients (71%) in the placebo group also achieved the goal (Citation57).
The ROADMAP study was also of note because of issues relating to safety. Overall rates of cardiovascular events were low in ROADMAP, but more deaths because of cardiovascular causes were seen in patients who received olmesartan than in placebo recipients (15 vs 3, p = 0.01). These findings are consistent with results of other studies indicating that excessive reduction of BP levels in hypertensive diabetic patients may even increase the risk of mortality. However, because the numbers of events involved were so low, the authors suggested that this result may simply have been down to chance. A subsequent investigation of the data from ROADMAP, and another study in diabetic patients, carried out by the US FDA found no safety concerns and concluded that benefits of olmesartan continue to outweigh its potential risks when used to treat patients with high BP (Citation58).
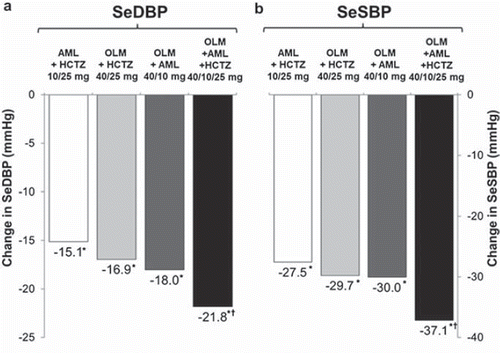
As discussed above, clinical studies with olmesartan demonstrate that triple combination therapy produces significantly better BP control. The efficacy of such a combination was highlighted in the recent Triple Therapy with Olmesartan Medoximil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients (TRINITY) study, which compared the antihypertensive efficacy of a triple combination of olmesartan (40 mg), amlodipine (10 mg) and HCTZ (25 mg) with each of the three possible dual combinations in a large group of patients with moderate-to-severe hypertension. Significantly greater reductions in seated DBP (primary efficacy parameter) and SBP () were observed with the triple combination compared with dual combinations (p < 0.001). A similar pattern of results was seen for changes in 24-h BP in a subgroup of patients in whom ABPM was used. At the end of the randomized double-blind treatment phase, the proportion of patients with seated SBP/DBP <140/90 mmHg was 69.9% in the triple combination treatment group, compared with 41.1–53.4% in the dual combination groups (p < 0.001 vs triple combination). Furthermore, triple combination therapy was well tolerated with similar rates of treatment-emergent adverse events (TEAEs) and drug-related TEAEs seen across the dual and triple combination groups (Citation19,Citation59,Citation60). More recently, two studies have used stepwise treat-to-target algorithms based upon olmesartan plus amlodipine plus HCTZ in hypertensive patients who were uncontrolled on initial monotherapy, and in hypertensive patients with diabetes. These two studies have shown that such an approach can produce high levels of goal rate achievement in these harder-to-treat patient types (Citation61,Citation62).
Figure 5. Change from baseline in least squares mean (a) seated diastolic blood pressure and (b) seated systolic blood pressure in patients randomized to 12 weeks’ treatment with a combination of amlodipine, hydrochlorothiazide and olmesartan, or each component dual combinations (Citation19). AML, amlodipine; HCTZ, hydrochlorothiazide; OLM, olmesartan; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
Conclusions
Combination therapy is an important factor in the treatment of hypertension, with the majority of patients requiring at least two agents to achieve target BP. The benefits of combination therapy on BP reduction are well established, and using RAS-blocker-based combinations, particularly those based upon ARBs, is logical, since these provide efficacy, good tolerability and are recommended in European guidelines. Clinical studies show that dual combinations of ARBs with amlodipine and HCTZ produce significantly better BP reductions and control rates than the constituent monotherapies. Within the ARB class, olmesartan stands out as being one that has been investigated in a considerable number of studies that are relevant to the modern concept of FDC therapy in terms of both dual and triple combination therapy. The availability of such single-pill FDCs has the potential to deliver strong antihypertensive efficacy with good tolerability and compliance.
Acknowledgements
Editorial assistance was provided by Amy McCallum (in Science Communications, a Wolters Kluwer business, Chester, UK) and contracted by Daiichi Sankyo Europe, the manufacturer of olmesartan/amlodipine. The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors, was responsible for content and editorial decisions and was involved at all stages of manuscript development. The author received no compensation related to the development of the manuscript.
Conflicts of interest: The author has received honoraria or travel support for lectures on hypertension from the following companies producing fixed-dose combination drugs: Daiichi Sankyo Europe, Berlin-Chemie AG, Menarini International, Boehringer Ingelheim GmbH, Krka, Servier Laboratories and Sanofi-Aventis.
References
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- European Society of Hypertension–European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1153.
- Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, . Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158.
- Zanchetti A, Grassi G, Mancia G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. J Hypertens. 2009;27:923–934.
- Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, . Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304: 61–68.
- Cushman WC, Evans GW, Byington RP, Goff DC, Jr., Grimm RH, Jr., Cutler JA, . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
- Wang YR, Alexander GC and Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167:141–147.
- Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, . Preserving renal function in adults with hypertension and diabetes: A consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661.
- Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, . Success and predictors of blood pressure control in diverse North American settings: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404.
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860.
- Wald DS, Law M, Morris JK, Bestwick JP and Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: Meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300.
- Law MR, Morris JK and Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.
- Erdine S. Compliance with the treatment of hypertension: The potential of combination therapy. J Clin Hypertens (Greenwich). 2010;12:40–46.
- Krousel-Wood M, Thomas S, Muntner P and Morisky D. Medication adherence: A key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–362.
- Schroeder K, Fahey T and Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732.
- WHO. Adherence to long-term therapies: Evidence for action. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. 2003. Accessed: 21 September 2010.
- Claxton AJ, Cramer J and Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310.
- Gupta AK, Arshad S and Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: A meta-analysis. Hypertension. 2010;55:399–407.
- Oparil S, Melino M, Lee J, Fernandez V and Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: The TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010; 32:1252–1269.
- Calhoun DA, Lacourciere Y, Chiang YT and Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: A randomized clinical trial. Hypertension. 2009;54:32–39.
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, . Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, . Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet. 2005;366: 895–906.
- PROGRESS Collaborative Study Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041.
- Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, . Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet. 2007;370: 829–840.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, . Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898.
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet. 2004;363:2022–2031.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, . A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil–Trandolapril Study (INVEST): A randomized controlled trial. JAMA. 2003;290:2805–2816.
- Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428.
- Law MR, Wald NJ, Morris JK and Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. BMJ. 2003;326: 1427.
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
- Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P and Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630.
- Schmieder RE. The role of fixed-dose combination therapy with drugs that target the renin–angiotensin system in the hypertension paradigm. Clin Exp Hypertens [Research Support, Non-U.S. Gov't Review]. 2010;32:35–42.
- Ram CV. Antihypertensive efficacy of angiotensin receptor blockers in combination with hydrochlorothiazide: A review of the factorial-design studies. J Clin Hypertens [Research Support, Non-U.S. Gov't Review]. 2004;6:569–577.
- Ram CV. Antihypertensive efficacy of olmesartan medoxomil or valsartan in combination with amlodipine: A review of factorial-design studies. Current medical research and opinion [Review]. 2009;25:177–185.
- Maillard MP, Wurzner G, Nussberger J, Centeno C, Burnier M and Brunner HR. Comparative angiotensin II receptor blockade in healthy volunteers: The importance of dosing. Clin Pharmacol Ther. 2002;71:68–76.
- Coltamai L, Maillard M, Simon A, Vogt B and Burnier M. Comparative vascular and renal tubular effects of angiotensin II receptor blockers combined with a thiazide diuretic in humans. J Hypertens. 2010;28:520–526.
- Mazzolai L, Maillard M, Rossat J, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade in normotensive subjects: A direct comparison of three AT1 receptor antagonists. Hypertension. 1999;33:850–855.
- Fabia MJ, Abdilla N, Oltra R, Fernandez C and Redon J. Antihypertensive activity of angiotensin II AT1 receptor antagonists: A systematic review of studies with 24 h ambulatory blood pressure monitoring. J Hypertens. 2007;25: 1327–1336.
- Hasler C, Nussberger J, Maillard M, Forclaz A, Brunner HR and Burnier M. Sustained 24-hour blockade of the renin– angiotensin system: A high dose of a long-acting blocker is as effective as a lower dose combined with an angiotensin-converting enzyme inhibitor. Clin Pharmacol Ther. 2005; 78:501–507.
- Jones MR, Sealey JE and Laragh JH. Effects of angiotensin receptor blockers on ambulatory plasma Renin activity in healthy, normal subjects during unrestricted sodium intake. American Journal of Hypertension. 2007;20:907–916.
- Smith DH, Dubiel R and Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: A comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5:41–50.
- Brunner HR, Stumpe KO and Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Invest. 2003;23:419–430.
- Neutel JM. Prescribing patterns in hypertension: The emerging role of fixed-dose combinations for attaining BP goals in hypertensive patients. Current Med Res Opin [Research Support, Non-U.S. Gov't Review]. 2008;24:2389–2401.
- Fogari R, Taddei S, Holm-Bentzen M, Baszak J, Melani L and Schumacher K. Efficacy and safety of olmesartan medoxomil 40 mg/hydrochlorothiazide 12.5 mg combination therapy versus olmesartan medoxomil 40 mg monotherapy in patients with moderate to severe hypertension: A randomized, double-blind, parallel-group, multicentre, multinational, phase III study. Clin Drug Investig. 2010;30:581–597.
- Oparil S, Chrysant SG, Kereiakes D, Xu J, Chavanu KJ, Waverczak W, . Results of an olmesartan medoxomil-based treatment regimen in hypertensive patients. J Clin Hypertens (Greenwich). 2008;10:911–921.
- Volpe M, Brommer P, Haag U and Miele C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: A randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009; 29:11–25.
- Rump LC and Sellin L. Combination therapy for hypertension: Focus on high-dose olmesartan medoxomil (40 mg) plus hydrochlorothiazide. Expert Opin Pharmacother. 2010;11: 2231–2242.
- Chrysant S, Melino M, Karki S, Lee J and Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30:587–604.
- Heagerty AM, Laeis P and Haag U. Olmesartan medoxomil/amlodipine (OLM/AML) provides 24-hour antihypertensive efficacy – additional effect by uptitration in patients with moderate-to-severe hypertension. J Hypertens. 2009;27 Suppl 4 S283.
- Chrysant SG, Weber MA, Wang AC and Hinman DJ. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17:252–259.
- Chrysant SG, Chavanu KJ and Xu J. Combination therapy with olmesartan medoxomil and hydrochlorothiazide: Secondary analysis of the proportion of patients achieving recommended blood pressure goals from a randomized, double-blind, factorial study. Am J Cardiovasc Drugs. 2009;9:241–251.
- Rump LC, Girerd X, Sellin L and Stegbauer J. Effects of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertension. J Hum Hypertens. 2010;Epub ahead of print.
- Kereiakes DJ, Neutel J, Stoakes KA, Waverczak WF, Xu J, Shojaee A, . The effects of an olmesartan medoxomil-based treatment algorithm on 24-hour blood pressure levels in elderly patients aged 65 and older. J Clin Hypertens (Greenwich). 2009;11:411–421.
- Neutel JM, Kereiakes DJ, Waverczak WF, Stoakes KA, Xu J and Shojaee A. Effects of an olmesartan medoxomil based treatment algorithm on 24-hour blood pressure control in patients with hypertension and type 2 diabetes. Curr Med Res Opin. 2010;26:721–728.
- Punzi H, Neutel JM, Kereiakes DJ, Shojaee A, Waverczak WF, Dubiel R, . Efficacy of amlodipine and olmesartan medoxomil in patients with hypertension: The AZOR Trial Evaluating Blood Pressure Reductions and Control (AZTEC) study. Ther Adv Cardiovasc Dis. 2010;4:209–221.
- Ram VS, Sachson RA, Qian C, Patel M and Stoakes KA. Effects of an amlodipine- and olmesartan medoxomil-based titration regimen in patients with hypertension, type 2 diabetes, and metabolic syndrome. J Clin Hypertens. 2010;12:A96.
- Haller H, Ito S, Izzo JL, Jr., Januszewicz A, Katayama S, Menne J, . Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New Engl J Med. 2011;364:907–917.
- US Food and Drug Administration. FDA Drug Safety Communication: Safety Review Update of Benicar (olmesartan) and cardiovascular events. http://www.fda.gov/Drugs/DrugSafety/ucm251268.htm; 2011.
- Chrysant S, Melino M, Fernandez V, Lee J and Heyrman R. Efficacy and safety of combination olmesartan medoxomil (OM)+amlodipine besylate (AML)+hydrochlorothiazide (HCTZ) in patients with hypertension: Analysis by age and gender. J Clin Hypertens. 2010;12Suppl 1:A30–A31.
- Izzo J, Melino M, Fernandez V, Lee J and Heyrman R. 24-hour efficacy and safety of full-dose, triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide. J Clin Hypertens. 2010;12 Suppl 1:A34–A35.
- Ram CV, Sachson R, Littlejohn T, Qian C, Shojaee A, Stoakes KA, . Management of hypertension in patients with diabetes using an amlodipine-, olmesartan medoxomil-, and hydrochlorothiazide-based titration regimen. Am J Cardiol. 2011;107:1346–1352.
- Weir MR, Hsueh W, Nesbitt SD, Littlejohn TJ, Graff A, Shojaee A, . A titrate-to-goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed-dose combinations of amlodipine and olmesartan medoxomil +/− hydrochlorothiazide. J Clin Hypertens. 2011;Published ahead of print (5 FEB 2011, DOI: 10.1111/j.1751-7176.2011.00437).