Abstract
Aim. To compare two strengths of a fixed drug combination (FDC) containing metoprolol XL and amlodipine (metoprolol/amlodipine 50/5; and metoprolol/amlodipine 25/2.5) with its components in hypertension. Methods. We conducted this multicentre, randomized, open-label, trial in Indian patients with hypertension (140–180 mmHg/90–114 mmHg) in 11 centres from nine cities. Eligible patients (n = 402) were randomized into one of five treatment groups (metoprolol XL 50 mg + amlodipine 5 mg, metoprolol XL 25 mg + amlodipine 2.5 mg, metoprolol XL 50 mg, metoprolol XL 25 mg or amlodipine 5 mg) and treated for 8 weeks with five follow-up visits to record blood pressure (BP) and clinical status. Results. At baseline, treatment groups were well balanced; mean ± SD BP was 154.87 ± 11.91/96.63 ± 6.97 mmHg. The greatest reduction in BP from baseline to 8 weeks was seen in the high-dose FDC group (23.61/14.91 mmHg; p < 0.001). The remaining 4 groups too demonstrated a significant reduction (p < 0.001): low-dose FDC − 22.29/ − 14.66; metoprolol 50, − 23.17/ − 13.37; metoprolol 25, − 18.41/ − 12.50 and amlodipine 5, − 23.01/ − 13.08. BP reductions by FDCs, however, were not statistically superior to monotherapies. Responder rates (sitting diastolic BP < 90 mmHg or reduction ≥ 10 mmHg) were 93% in the high-dose FDC group and 97% in the low-dose FDC group, and control rates (sitting BP < 140/90 mmHg) were 66% and 58%, respectively. These rates were higher than that seen in individual components. There were no reports of serious adverse events related to study medications. One each from the low-dose FDC and metoprolol 25 mg group discontinued because of adverse events. Conclusions. FDCs of metoprolol and amlodipine are effective and safe in mild to moderate hypertension.
Introduction
Hypertension is a major risk factor for cardiovascular and renal diseases. Untreated hypertension can reduce life expectancy by approximately 5 years (Citation1). Despite the importance of treating high blood pressure (BP) to reduce cardiovascular complications, most patients do not achieve the target blood pressure. The seventh Joint National Committee on prevention, detection, evaluation and treatment of high BP (JNC 7) recommends appropriate treatment of hypertension and to consider combination therapy to achieve and maintain the goal BP (Citation2). TheAntihypertensive and Lipid Lowering to prevent Heart Attack Trial (ALLHAT) supported the combination of antihypertensive therapy by reporting that irrespective of the initial antihypertensive agents; more than 40% of hypertensive patients require two or more drugs at the end of 4 years of treatment (Citation3).
Calcium-channel blockers inhibit the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Beta-blockers suppress renin release from kidney, reduce noradrenaline release from sympathetic terminals and with continued treatment, resistance vessels gradually adapt chronically to lower cardiac output that decreases total peripheral resistance. JNC 7 has recommended both of these drug classes as first line therapy for treating hypertension (Citation2).
Amlodipine is a long-acting calcium-channel blocker with an elimination half-life of 35–50 h. The long half-life makes it suitable for once a day dosing (in a fixed-dose combination). Metoprolol is the prototype of beta-1 cardio-selective blockers.
Randomized controlled trials have demonstrated that fixed drug combinations (FDCs) of calcium-channel blockers and beta-blockers have complementary antihypertensive effects with low rates of adverse effects (AEs) (Citation4–6). FDCs reduce the pill burden and may improve treatment adherence in a chronic disease like hypertension (Citation7,Citation8).
There are no published studies to date with a combination of metoprolol XL and amlodipine in essential hypertension. We conducted a randomized controlled open-multicentre study to compare the efficacy and safety of two FDCs of amlodipine and metoprolol with their individual components in Indian patients with essential hypertension. The primary objective of this study was to demonstrate that the high-dose FDC of metoprolol XL 50 mg and amlodipine 5 mg was superior to monotherapy with its individual components, metoprolol XL 50 mg and amlodipine 5 mg, in reducing systolic (SBP) and diastolic BP (DBP).
Patients and methods
The study protocol was approved by the National Regulatory Authority (Drugs Controller General of India) and by each of the institutional ethics committees. Written informed consent was obtained from all patients prior to screening and enrolment in the study. The trial was registered at clinicaltrials.gov, number NCT 00819104 and Clinical Trials Registry of India, CTRI/2008/091/000190, 04-02-209.
Patients
Eligible patients were 18–80 years of age with a SBP of 140–179 mmHg and DBP of 90–114 mmHg, documented during two office visits. Patients with DBP between 114 and 120 mmHg were also screened and included if the average of two DBP readings was between 90 and 114 mmHg. We excluded patients with secondary hypertension, prior history of cardiovascular events, grade 2 or grade 3 heart block, serum creatinine > 2.0 mg/dl, serum potassium < 3.0 mEq/l, hepatic enzymes > 2.5 times the upper limit of normal, or fasting blood glucose ≥200 mg/dl or <60 mg/dl. We also excluded patients with insulin-treated diabetes mellitus, bronchial asthma and other obstructive lung disease, previous enrolment in the present study and participation in another clinical study during the last 30 days.
Study design
MARS was a multicentre, randomized, open-label parallel trial conducted in 11 centres in nine cities across India between November 2008 and August 2009. Seven visits were required for each subject (both drug-naïve as well as patients on drug treatment) over a treatment period of 8 weeks. At the first visit, eligibility assessment, informed consent and the necessary investigations were carried out to screen the patient for essential hypertension. Before randomization, all patients underwent a clinical examination and recording of 12-lead electrocardiogram, heart rate and BP. We measured SBP and DBP of patients included in the study at all sites using a digital automatic BP monitor (Omron, model HEM-7080, Omron Health Care Co., Ltd, Kyoto, Japan). Laboratory measurements included haematology, routine and microscopic examination of urine, urine pregnancy test, clinical chemistry (liver function tests, serum electrolytes, blood urea, serum creatinine, glycosylated haemoglobin) and ultrasonogram of abdomen (to rule out secondary causes of hypertension).
If already on an antihypertensive medication, the patient underwent a washout period of at least 3 weeks before randomisation. Patients who stopped antihypertensive medication were monitored closely during the washout period for BP as well as for other symptoms at least three times, at intervals of 5–7 days (at the investigator's clinic or measured by a local physician and reported to the study physician by telephone). At the end of the washout period, all patients were re-assessed for eligibility.
Randomization and study procedures
St John's Research Institute (SJRI), Bangalore, generated the randomization sequence for MARS. Sites called SJRI by telephone to randomize an eligible patient in to one of five different groups. The five treatment groups were: high-dose FDC of metoprolol XL 50 mg and amlodipine 5 mg (metoprolol/amlodipine 50/5); low-dose FDC of metoprolol XL 25 mg and amlodipine 2.5 mg (metoprolol/amlodipine 25/2.5; both FDCs are bilayered tablets from AstraZeneca, India); metoprolol XL 50 mg (IPCA Pharma, India); metoprolol XL 25 mg (IPCA Pharma, India) and amlodipine 5 mg (Pfizer, India). Patients took the drug(s) once daily orally.
During the first visit, eligibility assessment and screening tests were carried out. Drug-naïve patients were randomized after eligibility assessment at the second visit. There were four on-treatment follow-up visits at 2, 4, 6 and 8 weeks. Laboratory parameters, electrocardiogram and urine pregnancy tests were repeated at the end of study medications at the sixth visit (8 weeks). The study drugs were stopped after 8 weeks. Further treatment was at the discretion of the treating physician. The subjects underwent a final follow-up visit (visit 7) at 12 weeks (4 weeks after stopping study medications) for safety assessment. Investigators were requested to recommend suitable lifestyle modifications at each visit. Increase in BP during the study period was managed appropriately by adding an appropriate rescue medication (other than a beta-blocker and calcium-channel blocker). Study drug compliance was assessed at each visit by pill count or the recording in the patient diary.
Efficacy assessment
The primary efficacy outcomes were the changes in sitting SBP and DBP between high-dose FDC of metoprolol 50 mg and amlodipine 5 mg and its individual components (metoprolol XL 50 mg and amlodipine 5 mg) from baseline. The secondary objectives of the study were to demonstrate that (i) the low-dose FDC of metoprolol XL 25 mg and amlodipine 2.5 mg is superior to monotherapy with metoprolol XL 25 mg and amlodipine 5 mg in reducing SBP and DBP, (ii) assess the number of responders (percentage of patients with sitting DBP < 90 mmHg or a reduction of sitting DBP ≥ 10 mmHg), (iii) assess the control rates (percentage of patients with sitting DBP < 90 mmHg and SBP < 140 mmHg) in each group, and (iv) assess the safety and tolerability of the high-dose as well as the low-dose combinations. The secondary efficacy end points were changes in SBP and DBP between low-dose FDC of metoprolol 25 mg and amlodipine 2.5 mg, and the other three treatment groups (metoprolol XL 25 mg, amlodipine 5 mg and the high-dose FDC of metoprolol/amlodipine 50/5), change in the heart rate, responder rate (responders were patients with sitting DBP < 90 mmHg or reduction in sitting DBP ≥ 10 mmHg (Citation9)) and control rate (defined as percentage of patients with sitting DBP < 90 mmHg and sitting SBP < 140 mmHg (Citation9)). BP was measured at all seven visits by the study physician. BP and heart rate were measured after a rest for 5 min. At least two sitting BP measurements, at an interval of 2 min were recorded. Two further readings were obtained 30 s and 2 min after standing to check for postural hypotension. The BP recorded for assessment was the mean of the two consecutive readings taken with the patient sitting down.
Safety assessment
Safety assessments included AEs, clinical chemistry, haematology, lipid and urine tests, physical examination and electrocardiographic findings.
Sample size
A total sample size of 300 patients with 60 patients in each arm would detect a sitting DBP difference of 5 mmHg (SD 8 mmHg), at a two-sided significance level of 0.05 with 80% power. We determined slightly higher sample sizes for the low doses as a smaller effect size was expected in its comparison with the monotherapy. We estimated final a total sample size of 400 patients after accounting for a 10% dropout rate.
Statistical analysis
Normality of the data were examined using Q–Q plots and SD. Data were described using mean and SD when normally distributed and median (inter quartile range, IQR) for non-normally distributed data. For the primary and secondary efficacy and safety analyses, we included all patients randomized who received at least one dose of study medication and who had completed at least one post-baseline assessment (modified intent to treat [mITT] analysis). The last observation carried forward method (LOCF) was used for patients who did not complete all follow-up visits.
The primary statistical analysis was a two-way analysis of covariance (ANCOVA) of change in BP with the baseline SBP or DBP as covariates and treatment group and study site as two factors. The three treatment groups considered in this analysis were metoprolol 50 mg + amlodipine 5 mg, amlodipine 5 mg and metoprolol XL 50 mg. We used the least-square mean changes in BP from baseline to assess the superiority of the FDC to the two individual components. We used the Hochberg's multiple testing step-up procedure as the post hoc method for pair-wise comparison of treatment groups. The same analysis was repeated in all study arms with a post hoc Dunnet test. We examined these objectives in a completer population, which included subjects who did not have any major protocol deviations and had end of study visit data. We used the paired-sample t-test to analyse change in BP from baseline to the end of study.
The responder and control rates between the treatment groups were compared using logistic regression models for BP with treatment group and centre as factors. All testing was two-sided and performed without adjustment for testing multiple parameters. Analysis was performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
Results
Patient allocation and baseline characteristics
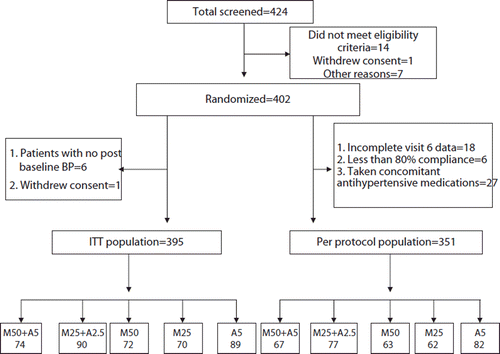
Of 424 patients screened, 402 (95%) were randomized. Among these, 74 patients were allocated to the high-dose FDC group, 90 to the low-dose FDC group, 72 to the metoprolol XL 50 mg group, 70 to the metoprolol XL 25 mg group and 89 to the amlodipine 5 mg group.
Baseline characteristics were comparable among the five treatment groups (). The mean age was 50.27 ± 11.4 years. Overall, 22 patients (5.57%) underwent a washout period before inclusion in the study.
Table I. Baseline characteristics of study participants (n = 395).
SBP and sitting DBP
Three hundred and ninety-five patients were included in mITT analysis (six patients did not have a post-baseline BP measurement and one patient withdrew consent soon after randomization).
The mean reduction in BP (± SD) measured at 8 weeks in the high FDC group (M50 + A5) was 22.58 ± 13.74 mmHg SBP and 14.4 ± 7.66 mmHg DBP. Mean reductions in other groups have been listed in . The reductions in BP across the five different groups were not statistically different. The reduction in BP by the two different FDCs was not statistically superior to monotherapies (). All groups of medications showed statistically significant (p < 0.001) reduction in BP from baseline.
Table II. Mean reduction in systolic (SBP) and diastolic blood pressure (DBP) across treatment groups.
Rate of fall in BP across various groups is shown in .
Responder rates
More patients on combination therapies (93% of patients in high-dose FDC and 97% in low-dose FDC) responded compared with monotherapies with metoprolol 50 (86%), metoprolol 25 (84%) or amlodipine 5 (87%) ().
Table III. Responder ratesa and control ratesb between treatment groups.
Control rates
More patients on the high FDC (66%) achieved BP control (< 140/90 mmHg) compared with low-dose FDC (58%), metoprolol 50 (56%), metoprolol 25 (56%) and amlodipine 5 (61%) ()
Safety
The three most common AEs overall were headache (4.3%), peripheral oedema (3.5%) and dizziness (2.3%) (). There was no change from baseline in blood glucose, total cholesterol and triglycerides across the five treatment groups. One patient each from low-dose FDC group and metoprolol 25 mg group discontinued from the study because of AEs (headache and allergic skin reactions respectively). High-dose FDC caused maximum reduction in heart rate (8 beats/min) and the least reduction was seen in the amlodipine 5 mg group (2 beats/min) (). None of the patients’ SBP fell below 90 mmHg and in three patients DBP fell below 60 mmHg.
Table IV. Rates of adverse events in different study groups.
Table V. Change in heart rate across treatment groups (per protocol population, n = 351).
Two patients experienced serious AEs (SAEs) during the study. Both of these were reported as not related to the study medications. One patient was hospitalized because of excessive alcohol consumption in the metoprolol 50 mg group and one patient in the metoprolol 25 mg group died of natural causes.
A per protocol analysis was carried among 351 patients (27 (6.7%) received concomitant antihypertensive drugs, 24 patients had incomplete data). Of the patients included in the per-protocol analysis, 67 were in the high-dose FDC group, 77 in the low-dose FDC group, 63 in the metoprolol XL 50 mg group, 62 in the metoprolol XL 25 mg group and 82 in the amlodipine 5 mg group. Details of patient disposition are presented in .
All groups in the per protocol analysis demonstrated significant reductions in BP from baseline (p < 0.001) but there was no significant difference across groups.
Discussion
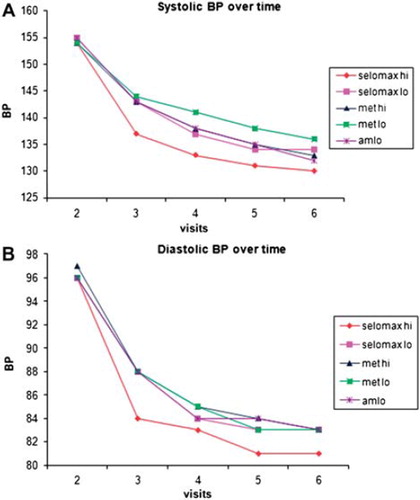
It is known that substantial decrease in cardiovascular complications can be achieved even with small reductions in BP. A 2 mmHg reduction in DBP is estimated to decrease the risk of coronary artery disease by 6% and stroke by 15% (Citation10). Analyses of pooled results of 3 double blind, placebo controlled studies of metoprolol CR/XL 50 mg once daily demonstrated a reduction of 8.4/4.8 mmHg in supine BP (Citation11). MARS is the first randomized, multicentre trial to assess the efficacy and safety of two doses of FDCs of amlodipine and metoprolol in hypertension. This study has shown that treatment with these FDCs produced robust reductions in both SBP and DBP (− 23.61/ − 14.9 and − 22.29/ − 14.66 respectively) in patients with mild to moderate hypertension. In direct comparison studies, felodipine/metoprolol decreased DBP to a greater extent than captopril with hydrochlorothiazide and nifedipine/atenolol but not with amlodipine alone (Citation12). The antihypertensive effect of the combination of felodipine and metoprolol occurs mostly in the first month of treatment with small additional decrease in BP in the second and third months and little additional decrease thereafter (Citation12). In MARS too, we observed a similar pattern ().
Figure 2. Mean sitting systolic blood pressure (A) and mean diastolic blood pressure (B) by treatment and visit.
Responder rates and control rates with antihypertensive medications is a direct indicator of treatment efficacy. In previous studies with felodipine/metoprolol BP control rates were between 63% and76% (Citation9). The study by Dahlof et al. (Citation13), with felodipine/metoprolol combination for 12 weeks, reported a responder rate of 90% and a control rate of 71%. A similar study by Hoffmann (Citation14), in 182 patients over 12 weeks, reported a BP reduction of 28/17 mmHg, with 98% responder rate and 73% control rate. In our study, after 8 weeks of treatment, a greater proportion of patients on combination therapies (93% of patients in high-dose FDC and 97% in low-dose FDC) responded compared with monotherapy with metoprolol or amlodipine (). Furthermore, a greater proportion of patients receiving high-dose FDC achieved a control of BP (< 140/90) (66%) compared with other treatment groups.
In MARS, low-dose and high-dose combinations of amlodipine and metoprolol were well tolerated with low rates of AEs (12 events and 16 events, respectively). Overall, headache and peripheral oedema were the most commonly reported AEs as seen in a previous study (Citation5). Types of AEs reported in our study were similar to those in previous studies with felodipine/metoprolol combinations (Citation13,Citation14). No drug-related SAEs were reported in both FDCs as well as in any of the other treatment groups.
Of 402 randomized patients, 20 patients (4.98%) discontinued participation for various reasons, which is lower than that seen in similar previous studies (Citation5). In this study, only six (1.5%) patients reported less than 80% compliance. Simplified, dosage regimens improve patient compliance especially when the dosage frequency is once daily (Citation12).
The objective of antihypertensive therapy is to reduce the hypertension-related cardiovascular mortality and complications. Adequate BP deduction is the key factor for preventing the mortality and morbidity. Successful antihypertensive therapy depends on the efficacy of the therapeutic agents and also its safety or its effect on the patient's well being. An important place for metoprolol/amlodipine combination in the management of hypertension can be established based on the finding from MARS.
MARS has some important strengths. It is the first multicenter, centrally randomized trial in Indian patients to evaluate high- and low-dose FDCs of amlodipine and metoprolol in hypertension. The discontinuation rates, overall, as well as AE related were lower than reported in previous studies (Citation5). The rates of hypotension were low and there were no reports of drug-related SAEs.
Our study has some limitations. We chose an open-label design for logistic reasons. As it was a short-term trial (8 weeks), we were not able to assess the long-term efficacy and adherence to this combination in a chronic disease such as hypertension. The observed reduction in BP did not demonstrate an important additive effect. The reasons for this are unclear. Long-term studies with a larger sample size may provide some insights.
Conclusion
MARS, the first multicenter, centrally randomized trial in Indian patients, demonstrated that both high- and low-dose FDCs of amlodipine and metoprolol are safe and effective BP lowering FDCs in hypertension. Both FDCs demonstrated clinically meaningful BP reductions and better responder rates compared with monotherapies. No SAEs were reported in both FDC groups and AE rates were low. Therefore, when a combination of metoprolol and amlodipine is indicated, these data from MARS provide an evidence-based option that is safe and effective.
Acknowledgements
The authors gratefully acknowledge the following staff for their substantial contribution to MARS. Study co-ordinators: Ms Freeda Xavier and Ms Preeti Girish, Division of Clinical Trials, SJRI, Bangalore. Study statisticians: Ms Nisha George and Ms Seena Thomas, Division of Clinical Trials, SJRI, Bangalore. Data base development team: Dr Tony Raj and Mr Valsan, Unit of Data Management and Informatics, SJRI, Bangalore. Dr Jitendra Sharma, Department of Pharmacology, St John's Medical College, Bangalore. Drug packaging and randomization team: Dr Mangala Rao, Dr Shivanagouda Patil, Dr Deepak Kamath (Department of Pharmacology, St John's Medical College, Bangalore), Ms Preeti Girish, Mr Rajan Chellom (Division of Clinical Trials, SJRI, Bangalore). Design of case record forms and study documents: Dr Leena N Kumar and Dr Jitendra Sharma, Department of Pharmacology, St John's Medical College, Bangalore. Data management team: Ms Freeda Xavier, Ms Preeti Girish, Mr Pankaj Verman, Ms Jalin James, Mr Manoj Sahoo, Mr Nabeel Basheer and Mr Kishore Kumar, Division of Clinical Trials, SJRI, Bangalore.
Sources of support
This study was sponsored by AstraZeneca Pharma Ltd, Bangalore, India. AstraZeneca also supplied the blood pressure recording equipment and drugs for this study.
Declaration of interest: Drs. Pais, Xavier and Sigamani have received research funds from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer and Cadila. Dr Sudhanshu Pandey, was an employee of AstraZeneca Pharma Ltd., when this study was designed and conducted. The sponsor played no part in the data management or analysis.
References
- Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women. Hypertension. 2005;46:280–286.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003; 289:2560–2572.
- ALLHAT Collaborative research group. Major cardiovascular events in hypertensive patients randomized to doxazocin vs chlorthalidone: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Am Med Assoc. 2000;283:1967–1975.
- Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Scherstén B. Randomized trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish Trial in Old patients with Hypertension-2 study. Lancet. 1999;354:1751–1756.
- Frishman WH, Hainer JW and Sugg J for the M-FACT Study Group. A factorial study of combination hypertension treatment with metoprolol succinate extended release and felodipine extended release results of the Metoprolol Succinate– Felodipine Antihypertension Combination Trial (M-FACT). AJH. 2006;19:388–395.
- Papademetriou V, Hainer JW, Sugg J, Munzer D for the ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. AJH. 2006;19:1217–1225.
- Dezii CM. A retrospective study of persistence with single pill combination therapy vs. concurrent two pill therapy in patients with hypertension. Manag Care. 2000;9 Suppl 9:S2–S6.
- Burnier M. Medication adherence and persistence as the cornerstone effective antihypertensive therapy. Am J Hypertension. 2006;19:1190–1196.
- Klein G, for the German felodipine study group. Long-term evaluation of the fixed combination of felodipine and metoprolol in the treatment of hypertension. Curr Ther Res. 1992;52:238–242.
- Cook NR, Cohen J, Hebert PR, Taylor Jo, Honnekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709.
- Westergren G, Olofsson B and Parlevliet K. Effective once-daily treatment of hypertension with low-dose, controlled release metoprolol. Curr Ther Res. 1994;55:142–148.
- Haria M, Plosker GL, Markham A. Felodipine/metorolol, a review of the fixed dose controlled formulation in the management of essential hypertension. Drugs. 2000;59:141–157.
- Dahlof B, Jonsson L, Borgholst O, Ekblad G, Engstrand C, Grundestam I, . Improved antihypertensive efficacy of the felodipine metoprolol extended release tablet compared with each drug alone. Blood Press. 1993;2 Supp l:37–45.
- Hoffmann J. Comparison of a felodipine–metoprolol combination tablet vs each component alone as antihypertensive therapy. Blood Press. 1993;2 Suppl 1:30–36.