Abstract
Recent evidence indicates that skewed immune responses occur in patients with essential hypertension (EH), which may be involved in the development of EH. This study aimed to evaluate the plasma levels of immune complexes (IC), procollagen type I collagen carboxypropeptide (TICP), and von Willebrand factor (vWF) in EH patients. A total of 121 patients with EH were enrolled in this study (EH group). Thirty healthy volunteers were recruited to serve as the control group. The plasma levels of circulating IC (CIC), vWF and procollagen TICP were measured. Multiple linear regression assays were performed with the data to determine the correlation between EH and these parameters. The results showed that compared with the healthy control group, the plasma levels of CIC, vWF and TICP in the EH group were significantly higher than that in healthy control group, which was positively correlated with EH. The levels of CIC were also positively correlated with vWF and TICP in plasma. The results suggest that skewed immune responses exist in patients with EH.
Introduction
Although the etiology of essential hypertension (EH) is not fully understood, the pathophysiological changes of EH have been noted that the increase in arteriole tone plays a critical role in EH. Under a physiological condition, the kinetic balance between parasympathetic and sympathetic nerves maintains the blood pressure at optimal levels. The insufficient parasympathetic tone or hypersympathetic tone is suggested as the essential cause of EH (Citation1). However, the regulatory mechanism on the parasympathetic/sympathetic tone is not yet completely understood.
The potential role of immune imbalance in the development of EH has been noted in recent years (Citation2). A number of studies using animal models or human subjects indicate that immunomodulation is deranged in patients with EH (Citation2). Several inflammatory markers, including C-reactive protein (CRP) (Citation3,Citation4), interleukin (IL)-6 (Citation5) and IL-10 (Citation6) have been noted to be elevated in individuals with EH. The total immunoglobulin (Ig)G levels are also significantly higher in EH patients that is correlated to the elevated blood pressure level as well as the extent of the vascular wall damage (Citation7). Such immune abnormality may play an important role in mediating injury on endothelium of arterioles (Citation8).
Immune complexes (ICs), composed of antigens and antibodies, play a pivotal role in inflammatory responses. They exert their actions through stimulating and cross-ligating cellular receptors on macrophages and neutrophils, thereby stimulating phagocytosis leading to cellular immune responses (Citation9), such as an increase in endothelial permeability (Citation10), inducing leukocyte to migrate into tissue (Citation11) and inducing an inflammatory response in the local tissue (Citation12). However, it remains to be further understood whether a similar change of ICs occurs in EH.
The von Willebrand factor (vWF) is regarded as a marker of endothelial cell damage/dysfunction. An elevated plasma level of vWF is related to adverse cardiovascular outcomes (Citation13). Although ICs have been shown to induce leukocyte-endothelial cell interactions leading to endothelial cell damaged in animal models (Citation5), it remains to be further understood whether the circulation IC has a connection with plasma vWF in humans with EH.
Type I collagen is the major component of intracellular collagen in the cardiovascular system. Type I collagen carboxypropeptide (TICP) is a marker of type I collagen synthesis. An elevated TICP level was observed in EH patients, especially in those with the vascular endothelium dysfunction (Citation14). An overactivated collagen synthesis and thereby excessive fibrosis has been proposed as one of the underlying mechanisms in myocardial remodeling (Citation15). However, how the fibrosis is initiated in the cardiovascular system that may contribute to EH remains largely unknown.
Based on the information above, we hypothesize that ICs in the circulation may play a role in the pathogenesis of EH. To test the hypothesis, we evaluated the plasma levels of IC in a group of patients with EH. Indeed, high levels of IC were detected in EH patients.
Materials and methods
Study population
Patients who met the criteria of 1999 WHO/ISH blood pressure classification (Citation16) for the diagnosis of EH presented in the outpatient clinic of Chinese PLA General Hospital between January 2005 and December 2007 were enrolled in this study. The inclusion criteria were: patients were aged between 35 and 75 years, had a history of hypertension for more than 2 years, were not currently taking or not regularly taking anti-hypertensive medications, and had no history of infection in the preceding 2 weeks. The subjects with any of the following conditions were excluded: atherosclerotic diseases, other primary heart diseases, moderate to severe valvular regurgitation, diabetes, liver fibrosis, pulmonary fibrosis, multiple injury, connective tissue disease, secondary hypertension, alcoholic, cancer or chronic infectious diseases.
A total of 121 patients were included in the EH group and 30 normotensive volunteers in the control group (demographic data of these subjects as shown in . Among the EH group, 63 patients were male and 58 were female, with age ranging from 35 to 75 years; in the 30 normotensive controls group, 15 were male and 15 were female, with age ranging from 35 to 74 years; There were no significant differences in gender, age distribution and body mass index between these two groups ().
Table I. Demographic data.
Blood biochemical determinations
All study participants fasted for 12 h. Fasting blood samples were obtained from peripheral veins early in the morning. The samples were collected into EDTA-coated tubes and were immediately cooled on ice and centrifuged at 1500g at 4°C for 10 min. The supernatant was stored at −20°C until further analyses. The plasma was separated within 6 h and kept frozen at −20°C until further analysis.
Circulating immune complex (CIC). Plasma CIC was quantified by using CIC reagent kit (Dade Behring Marburg GmbH, Marburg, Germany). The assay was performed according to the protocol provide by the manufacturer. N latex CIC reagent consists of lyophilisate particle coated with human complement factor C1q; on reconstitution of the reagent as instructed, the concentration of suspended particles is optimal for agglutination measurement by nephelometry.
Immunoglobulin G (IgG). Plasma IgG level was measured using an IgG reagent kit (Dade Behring Marburg GmbH).
Serum cytokines. Peripheral blood samples were obtained from each subject; the sera were separated to determine the levels of the inflammatory cytokines by enzyme-linked immunosorbent assay (ELISA) with commercial ELISA kits (all kits were purchased from R&D Systems, Shanghai, China) following manufacturers’ instructions.
Both plasma IgG and CIC were measured by rate nephelometry (Dade Behring BN II Nephelometer, Marburg, Germany).The assay protocol in the instruction manual and current software version of the BN II nephelometer system was followed. All the steps are performed automatically by the system quality control. The intra-assay coefficients of variation (CV) were less than 3.9% and inter-assay CVs were less than 5.1%.
High-sensitivity C-reactive protein (hs-CRP). Hs-CRP was quantified by using an N Latex CRP assay kit (Dade Behring GmbH) on a Behring Nephelometer II (BN II, Dade Behring GmbH). The assay was performed according to the protocol provided by the manufacturer. The intra- and inter-assay CVs were less than 4%.
von Willebrand factor (vWF). The plasma vWF level was measured by ELISA using commercial polyclonal antisera (Sun Biotechnology Ltd, Shanghai, China). The assay was performed according to the manufacturer's instructions.
Type I collagen carboxypropeptide (TICP). TICP was determined by electrochemiluminescence using a Roche automatic immune analyzer. The assay kit was provided by Roche ltd (Roche Diagnostics Limited, Shanghai). The intrassay and interassay variations were CVs were < 4%.
Statistical analysis
All data are expressed as mean ± standard deviation and were analysed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). After distribution testing, differences between group means were analyzed by Student's t-test, as appropriate, for different variances. A multiple linear regression analysis was performed to evaluate the association between EH and the parameters of CIC, vWF and TICP. The level of statistical significance was set at a p-value ≤ 0.05 for all statistic analysis.
Results
Plasma levels of CIC are increased in patients with EH
Considering the immune inflammation might be associated with the pathogenesis of EH, we measured the plasma levels of CIC in patients with WH. As shown in (A), the plasma CICs were detected in both EH patients and healthy controls, but the CIC levels were significantly higher in EH patients than that in healthy controls ().
Figure 1. Plasma levels of circulating immune complex (CIC), von Willebrand factor (vWF), high-sensitivity C-reactive protein (Hs-CRP) and type I collagen carboxypropeptide (TICP). Blood samples were collected from 121 essential hypertension (EH) patients and 30 healthy subjects and analyzed by enzyme-linked immunosorbent assay (ELISA). Bars indicate the plasma levels of (A) CIC, (B) vWF and (C) TICP. Data were presented as mean ± SD. *p < 0.01, compared with healthy subjects.
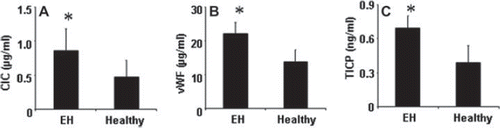
Plasma vWF levels are increased in EH patients
To investigate the potential role of vWF in the pathogenesis of EH, we measured the plasma levels of vWF in EH patients. The ELISA data showed that higher levels of vWF were detected in EH patients than that in healthy controls ().
Higher levels of TICP are detected in EH patients
We also measured the plasma levels of TICP in EH patients. The results showed that plasma TICP levels were markedly higher in EH patients than that in healthy controls ().
Serum inflammatory cytokines
The levels of serum inflammatory cytokines were measured for both healthy subjects and EH patients. As shown by ELISA, the levels of IL-6 and IL-10 were significantly higher in EH patients than that in healthy controls ().
Table II. Serum cytokine levels.
Multiple linear regression assays
We next performed multiple linear regression assays with the data in . The results showed a positive correlation was detected between EH and CIC (r = 0.88, p < 0.01), or vWF (r = 0.81, p < 0.01), or TICP (r = 0.66, p < 0.01). We further analyzed the correlation between CIC and other two parameters (vWF and TICP). The results also showed a positive correlation between CIC and vWF (r = 0.75, p < 0.01), or TICP (r = 0.52, p < 0.01). Furthermore, a positive correlation was also noted between plasma TICP and vWF (r = 0.45, p < 0.01) in the EH group.
Discussion
The data from this study indicate that the levels of CIC, vWF and TICP are significantly higher in patients with EH than that in healthy controls. The parameters of CIC, vWF and TICP are also positively correlated with EH. In order to check the inflammatory status, we measured the serum levels of several inflammatory cytokines. The results showed that a significant difference was detected in IL-6 and IL-10 between healthy subjects and EH patients, indicating there is detectable inflammation in the subjects.
In our study, an elevated plasma non-specific CIC level was observed in patients with EH. ICs are composed of antigens and antibodies (including different types of autoantibodies), which are important for inflammatory responses and elimination of complexed antigens. The elevated plasma CICs levels suggest that abnormal immune responses may exist in EH patients that promote CIC forming. Others also reported that increase in CICs in acute stroke patients that might be a result of liberation of chlamydial antigens into the circulation from injured areas of the vasculature (Citation17). Of course, the results do not exclude that the CIC may be released from other sources in the body. Mustafa et al. (Citation18) propose that high CIC levels may be a strong predictor of future cardiovascular events in patients with coronary heart disease. Because ICs can quickly induce inflammatory responses and increase microvascular permeability, long-term chronic elevation of CICs levels may mediate endothelial cellular damage activated by complements resulting in vascular dysfunction (Citation19). Therefore, high CICs levels may be a strong risk factor in vascular damage in EH patients.
In this study, we also found that the levels of vWF, a sensitive parameter of endothelial cell damage, were increased in EH patients compared with that in normotensive controls. It was found about 20 years ago that there was a relationship between vWF and hypertension (Citation20). The vWF was also higher in those patients with uncontrolled hypertension than in those whose hypertension was controlled (Citation21). Some studies showed that with blood pressure fluctuations and inadequate control, microvascular damage may be persistent in patients with EH. We found that vWF was also correlated with CIC, implying endothelial cell damage in these EH patients. This implies that immune responses may be involved in the endothelial cell damage in EH. Considering that high levels of ICs were detected in EH patients, the ICs may play an important role in endothelial cell damage because ICs have the ability to increase vascular permeability and induce substantial leukocyte adhesion (Citation22). The present data also reveal a positive correlation between CIC and vWF; thus, elevated CIC levels may be involved in the vascular damage in EH patients.
Fibrosis plays an important role in cardiac diastolic dysfunction and blood vessel dysfunction (Citation23). The collagen of the cardiovascular system mainly consists of type I collagen more than 80%. The increased production of TICP can be a useful marker of stimulated fibrogenesis in arterial hypertension. Serum TICP concentration was correlated directly with a collagen volume fraction, and was a highly sensitive and specific parameter in the identification of severe myocardial fibrosis in hypertension (Citation24). In the present study, we also found that TICP was increased in EH patients compared with that in normotensive controls, which is in line with published data (Citation24). Some factors may be involved in synthesis of collagen of EH. Firstly, the blood pressure overloads induce activated collagen synthesis (Citation24). Secondly, immune mechanism participated to collagen synthesis. Many immune factors can promote collagen synthesis, thereby resulting in myocardial fibrosis (Citation25), such as monocyte chemotactic protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1) and transforming growth factor (TGF). These factors have been linked with myocardial fibrosis (Citation26). Therefore, abnormal immunity may play an important role in facilitating myocardial fibrosis in EH. The increases in TICP levels are in parallel with CIC as observed in our study suggest that the immune response may affect the pathological progression of myocardial fibrosis and collagen synthesis of the vessel. Others also published supportive data (Citation27). Although it is unclear whether this inflammatory mechanism simply accompanies collagen synthesis or is only a consequence of end-organ damage caused by hypertension, the mechanisms by which CICs affect TICP warrants further investigation. The modulation of CICs and other inflammatory factors may help to regulate collagen synthesis and improve myocardial fibrosis in EH. In this study, we disclosed that vWF was also positively correlated with plasma TICP. The increases in plasma vWF levels can be resulted from endothelial cell damage and may also induce collagen synthesis in situ (Citation28).
In summary, elevated levels of CIC, vWF and TICP were detected in EH patients. These parameters are positively correlated with EH. The results indicate that skewed immune responses may be important in the development of EH.
Acknowledgment
This study was supported by a grant from the National Natural Science Foundation of China
Conflict of interest: The authors do not have any conflict of interest to declare.
References
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, . Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175.
- Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Ann N Y Acad Sciences. 2009;1179:153–166.
- Kurata M, Okura T, Irita J, Enomoto D, Nagao T, Jotoku M, . The relationship between osteopontin and adiponectin in patients with essential hypertension. Clin Exp Hypertens. 2010;32:358–363.
- Okura T, Jotoku M, Irita J, Enomoto D, Nagao T, Desilva V, . Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol. 2010;14:584–588.
- Yu X, Yang Z, Yu M. Correlation of tumor necrosis factor alpha and interleukin 6 with hypertensive renal damage. Ren Fail. 2010;32:475–479.
- Timasheva Y, Nasibullin T, Zakirova A, Mustafina O. Association of interleukin-6, interleukin-12, and interleukin-10 gene polymorphisms with essential hypertension in Tatars from Russia. Biochem Genetics. 2008;46:64–74.
- Garrido-Sánchez L, García-Fuentes E, Cardona F, Rojo-Martínez G, Soriguer F, Tinahones FJ. Anti-oxidized LDL antibody levels are reduced in women with hypertension. Eur J Clin Invest. 2009;39:800–806.
- Ishmael JE, Löhr CV, Fischer K, Kioussi C. Localization of myosin II regulatory light chain in the cerebral vasculature. Acta Histochemica. 2008;110:172–177.
- Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, . A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186:4994–5003.
- Basu A, Chaturvedi UC. Vascular endothelium: The battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299.
- Mersich AT, Miller MR, Chkourko H, Blystone SD. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton. 2010;67:573–585.
- Bartoloni E, Alunno A, Luccioli F, Moscatelli S, Biscontini D, Santoboni G, . Atherosclerotic vascular damage and rheumatoid arthritis: A complex but intriguing link. Expert Rev Cardiovasc Ther. 2010;8:1309–1316.
- Louden C, Brott D, Katein A, Kelly T, Gould S, Jones H, . Biomarkers and mechanisms of drug-induced vascular injury in non-rodents. Toxicologic Pathol. 2006;34:19–26.
- Lopez B, Querejeta R, Varo N, Gonzalez A, Larman M, Martinez Ubago JL, . Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104:286–291.
- Mori T, Kai H, Kajimoto H, Koga M, Kudo H, Takayama N, . Enhanced cardiac inflammation and fibrosis in ovariectomized hypertensive rats: A possible mechanism of diastolic dysfunction in postmenopausal women. Hypertens Res. 2011;34:496–502.
- Cuspidi C, Meani S, Salerno M, Severgnini B, Fusi V, Valerio C, . Cardiovascular risk stratification according to the. 2003 ESH‐ESC guidelines in uncomplicated patients with essential hypertension: Comparison with the 1999 WHO/ISH guidelines criteria. Blood Press. 2004;13:142–151.
- Tarnacka B, Gromadzka G, Czlonkowska A. Increased circulating immune complexes in acute stroke: The triggering role of Chlamydia pneumoniae and cytomegalovirus. Stroke. 2002;33:936–940.
- Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–2581.
- Lukacs NW, Glovsky MM, Ward PA. Complement- dependent immune complex-induced bronchial inflammation and hyperreactivity. Am J Physiol Lung Cell Mol Physiol. 2001;280:L512–L518.
- Lip GYH, Edmunds E, Martin SC, Jones AF, Blann AD, Beevers DG. A pilot study of homocyst(e)ine levels in essential hypertension: Relationship to von Willebrand factor, an index of endothelial damage. Am J Hypertens. 2001;14:627–631.
- Spencer CGC, Gurney D, Blann AD, Beevers DG, Lip GYH. Von Willebrand factor, soluble P-selectin, and target organ damage in hypertension: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Hypertension. 2002;40:61–66.
- Sunderkötter C. Vasculitis of small blood vessels – Some riddles about IgA and about the complexity of transmigration. Exp Dermatol. 2009;18:91–96.
- Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, . Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res. 2006;29:711–718.
- González A, López B, Ravassa S, Beaumont J, Arias T, Hermida N, . Biochemical markers of myocardial remodelling in hypertensive heart disease. Cardiovascular Res. 2009;81:509–518.
- Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104.
- Kuwahara F, Kai H, Tokuda K, Niiyama H, Tahara N, Kusaba K, . Roles of intercellular adhesion molecule-1 in hypertensive cardiac remodeling. Hypertension. 2003;41:819–823.
- Booz GW. Impact of T lymphocytes on cardiac remodeling in hypertension: More questions than answers. Hypertension. 2006;48:31–32.
- Rocnik EF, Chan BM, Pickering JG. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest. 1998;101:1889–1898.