Abstract
Background. Left atrial (LA) enlargement is a powerful risk factor for cardiovascular diseases; little information is available about its prevalence and correlates in subjects free of overt cardiac disease seen in echocardiographic practice. Aim. We evaluated the prevalence of LA enlargement (LAE) and the relationship with left ventricular (LV) mass and diastolic function in subjects with preserved LV systolic function referred to an echocardiographic study for routine clinical indications. Methods. 1104 subjects (mean age 58 ± 16 years, 46% men, 57% hypertensives) underwent a comprehensive echo-Doppler examination. LAE and LV hypertrophy (LVH) were defined as LA volume index (LAVI) >29 ml/m2 and LV mass index (LVMI) >50 g/h2.7, respectively. Abnormalities of LV relaxation and LV filling were diagnosed by age-related thresholds of lateral annular velocity (Ei) and by early mitral flow velocity to Ei ratio (E/Ei) ≥16, respectively. Results. Overall, 10% of echocardiographic examinations fulfilled the criteria for LAE, 46% for LVH, 45% for altered LV relaxation and 5% for altered LV filling index. LVH progressively increased from 25% to 75% across LAVI quartiles. More patients in the highest quartile exhibited abnormal indexes of LV relaxation and LV filling compared with lower quartiles. In multivariate analysis, LV mass index (β = 0.408), age (β = 0.188), E/Ei ratio (β = 0.140) and Ei (β = 0.140) emerged as major correlates of LAE (p at least <0.01 for all). Conclusions. LAE is a frequent finding in patients with preserved systolic function seen in current practice; this abnormality is strongly related to LVH and to diastolic dysfunction. Early detection of LAE may identify patients at higher cardiovascular risk and promote appropriate prevention strategies.
Introduction
Left atrial enlargement (LAE) is regarded as a reliable marker of increased cardiovascular risk in the general population and in patients with systemic hypertension and heart disease (Citation1,Citation2).
Cross-sectional studies have shown that LAE is related with unfavorable clinical and echocardiographic correlates including diabetes, obesity, metabolic syndrome, sleep apnea, hypertension, aging, left ventricular hypertrophy (LVH), systolic and diastolic dysfunction (Citation3–8).
Longitudinal studies have consistently demonstrated that LAE, as assessed by a single left atrial (LA) diameter or the more accurate LA volume (LAV), is an independent predictor of cardiovascular death, stroke, heart failure, atrial fibrillation and myocardial infarction (Citation9–12).
Recently, LAV has been shown to predict death and cardiovascular outcomes in patients with end-stage renal disease independently of validated markers of risk such as LVH and depressed left ventricular (LV) systolic function (Citation13).
Whether abnormalities in LA structure can be reverted and lead to an improvement in cardiovascular prognosis is less clear. In the Losartan Intervention for Endpoint Reduction in Hypertension Trial (LIFE), a reduction in LA diameter (LAD) during follow-up was related to LVH regression and to a lower incidence of new-onset atrial fibrillation or mitral regurgitation (Citation14).
Despite growing evidence that LAV is a relevant cardiac phenotype for cardiovascular risk stratification and monitoring, a few studies have analyzed the prevalence of this phenotype and its correlates in echocardiographic practice (Citation15).
Thus, the aim of the present study was to investigate this issue focusing on the relationship between LAV, LV structure and diastolic function in subjects with preserved cardiac function referred for routine echocardiographic examination by their practitioners to a single outpatient echo- laboratory.
Methods
A total of 1161 consecutive individuals, referred to the outpatient echocardiographic service of the Istituto Auxologico Italiano (Meda, Italy) by their general practitioners during a 15-month period from February 2009 to April 2010, were enrolled in the study. Main exclusion criteria were: (i) impaired LV ejection fraction (<40%); (ii) previous myocardial infarction or coronary bypass history; (iii) significant cardiac valve disease (regurgitation >1+ at Doppler examination, stenosis of any degree, presence of prosthesis and mitral annulus calcification); (iv) atrial fibrillation; (v) left bundle branch block.
After an informed consent had been obtained, patients’ demographic data, medical history and medications were collected at the echo-lab by a questionnaire administered by the attending physician. Obesity was identified by the 1998 National Institutes of Health classification as body mass index ≥30 kg/m2. High blood pressure (BP) was defined as systolic BP (SBP) ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg in untreated subjects or current antihypertensive therapy regardless BP values. Diabetes mellitus was defined by patient's self-report.
Measurements
Body weight was recorded to the nearest 0.1 kg using a calibrated electronic scale with the subjects wearing indoor clothing without shoes. Height was recorded to the nearest 0.5 cm using a standardized wall-mounted height board. Body mass index was computed as weight in kg divided by the squared height in meters.
Clinic BP was measured by mercury sphygmomanometer with appropriate sized cuffs; measurements were performed after the subjects had been resting for 3–5 min in the sitting position. Three measurements were taken from the non-dominant arm at 1-min intervals and the average was used to define patient's representative values.
Echocardiography
Echo and Doppler examinations were performed according to a standardized protocol. In brief, M-mode, two-dimensional and Doppler echo examinations were carried out with a commercially available instrument (Vivid 7, GE, Horten, Norway).
Briefly, end-diastolic (d) and end-systolic (s) LV internal diameters (LVID), interventricular septum thickness (IVS) and posterior wall thickness (PW) were measured from two-dimensionally guided M-mode tracings recorded at a speed of 50–100 cm/s, during at least three consecutive cycles according to the Penn convention. Relative wall thickness (RWT) was defined by the ratio of PW plus IVS thickness to LVIDd; LV mass was estimated by Devereux's formula {1.04[(IVSd + PWd + LVIDd)3− LVIDd3] − 13.6} (Citation16) and indexed to height to the allometric power of 2.7. LV ejection fraction was measured from the four-chamber apical projection in the standard fashion from LV end-diastolic and LV end-systolic volume.
LAD was determined at end-systole in the parasternal long-axis view, by the leading edge- to-leading edge maximal distance between end- systolic posterior aortic root wall and posterior LA wall; LAD was indexed to body surface area (Citation17).
LAV was measured at end-systole in the apical 4-chamber view by planimetry. A straight line connecting both sides of mitral valve attachment to the valve ring was taken as the superior border of the atrial outline; atrial appendage and pulmonary veins were carefully excluded. LAV was calculated by the biplane method of discs according to Simpson rule and indexed to body surface area (LAVI) (Citation17).
Conventional Doppler measurements
LV filling was assessed by recording mitral flow by standard pulsed Doppler technique in apical four-chamber view; the following parameters were considered: early diastolic peak flow velocity (E), late diastolic peak flow velocity (A), their ratio (E/A) and E wave deceleration time (from peak E-wave to baseline).
Tissue Doppler measurements
Tissue Doppler imaging of the mitral annulus movement was obtained from the apical four-chamber view. A 2.0-mm sample volume was sequentially placed at lateral and septal annular sites. Filters and gains were adjusted to minimize background noise and maximize tissue signal. Analysis was performed for early (Ei) and late (Ai) diastolic peak velocities and their ratio (Ei/Ai). LV filling index was determined by the ratio of transmitral flow velocity to annular velocity (E/Ei).
Reproducibility of echo-Doppler measurements
Intra-observer and inter-observer coefficients of variation calculated in a subsample of 107 consecutive patients (9.6% of the total study population) were as follows: 7.4% and 8.6% for LV mass index, 5.9% and 7.9% for LAVI, 5.1% and 6.3% for E velocity, 4.8% and 6.1% for A velocity, 5.0% and 5.9% for Ei velocity, and 5.3 and 6.1% for Ai velocity, respectively.
Definition of cardiac phenotypes
LVH was defined as LV mass index >50 g/h2.7 in both genders (Citation18). Patterns of abnormal LV geometry were defined as follows: (i) LV concentric remodeling (normal LV mass index combined with RWT ≥0.43); (ii) eccentric LVH (increased LV mass index combined with RWT <0.43); concentric LVH (increased LV mass index combined with RWT ≥0.43) (Citation17).
LAE was defined as LAVI >29 ml/m2 in both genders (Citation17), altered LV relaxation as lateral Ei velocity <10 cm/s in subjects 45–54 years old, <9 cm/s in subjects 55–65 years old and <8 cm/s in subjects older than 65 years (Citation19). Finally, LV filling index was considered altered for E/Ei ≥16 (Citation20).
Statistical analysis
Statistical analysis was performed by SAS System (version 6.12; SAS Institute Inc., Cary, NC, USA) and was mostly descriptive; values were expressed as means ± SD or as percentages. Mean values have been compared by Student's t-test for independent samples and categorical data analyzed by chi-square test or Fisher's exact test when appropriate. The strength of correlation between variables was tested by linear correlation analysis and multiple regression analysis. A p-value <0.05 was considered statistically significant.
Results
A total of 1104 out of 1161 patients (593 women, 511 men) fulfilled all study criteria and constituted the study population. Major indications for echocardiographic assessment were: arterial hypertension (33%), palpitations, pre-syncope or syncope (21%), suspected valve disease or murmurs (14%), dyspnea (9%), chest discomfort (8%) and other reasons (15%).
Overall, 57% of the study sample were hypertensives, 27% were obese and 7% had type 2 diabetes mellitus; 46% fulfilled the criteria for LVH 10% for LAE (), 45% for altered LV relaxation and 5% for altered LV filling index (see Methods).
Table I. Demographic and clinical characteristics of the study population (n = 1104).
shows demographic and clinical characteristics according to LAVI quartiles. Age, systolic and diastolic BP, body mass index as well as prevalence of obesity, hypertension, type 2 diabetes and antihypertensive, glucose-lowering, lipid-lowering, anti-platelet and anti-coagulant drugs showed a progressive increase across LAVI quartiles. This was not the case for gender, body surface area, heart rate and current smoking.
Table II. Demographic and clinical characteristics of the study population categorized in quartiles of left atrium volume index (LAVI).
Echocardiographic parameters are shown in . Mean values of LV internal diameters, wall thickness, absolute and indexed LV mass, mitral deceleration time and E/Ei ratio showed a progressive increase across the LAVI groups; on the contrary, LV ejection fraction, E/A, Ei and Ei/Ai values showed an opposite trend.
Table III. Echocardiographic variables of the study population categorized in quartiles of left atrium volume index (LAVI).
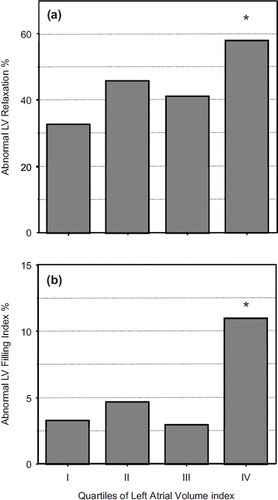
When alterations in LV structure and geometry as well as in LV filling index were analyzed as categorical traits across LAVI quartiles the following findings were observed: (i) LVH progressively increased from 25% to 75%; concentric LVH was prevalent over the eccentric type across all groups (); (ii) abnormal LV relaxation and LV filling indexes were present in a higher portion of patients in the upper LAVI quartile (). Differences persisted after adjustment for several confounders, such as age, gender, body mass index, BP, heart rate and anti-hypertensive drugs.
Figure 1. Prevalence of concentric (white bars) and eccentric (black bars) left ventricular hypertrophy (LVH) according to quartiles of left atrial volume index. *p < 0.01 IV vs III, II and I.
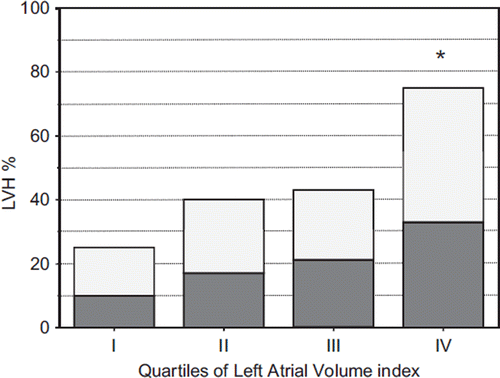
Figure 2. Prevalence of abnormal left ventricular (LV) relaxation and LV filling index according to quartiles of left atrial volume index. *p < 0.01 IV vs III, II, I.
A significant difference in the prevalence of LVH (32% vs 65%, p < 0.01), LAE (3.7% vs 18.1%, p < 0.01) and abnormal LV filling index (1.5% vs 12.0, p < 0.01) was found between younger and older subjects (< 5 and > 65 years).
In additional analyses, LAE, defined either according to single-dimension criterion (i.e. antero-posterior diameter >24 mm/m2) (Citation17) or to volume index (i.e. LAVI >24ml/m2) (Citation11) was detected in 18% and 21% of the whole sample, respectively.
Correlation analyses
In the whole population, LAVI, in ranking order, was positively associated with LVMI (r = 0.43), E/Ei ratio (r = 0.29), age (r = 0.28), clinic SBP (r = 0.24), Ei (r = 0.20), aortic root diameter (r = 0.17), body mass index (r = 0.16), LV RWT (r = 0.11) and inversely associated with LV ejection fraction (r = 0.15), heart rate (r = 0.12), Ei/Ai ratio (r = 0.10), E/A ratio (r = 0.09) (p < .001 for all).
When these variables were tested in multiple regression analyses, LVMI (β = 0.408), followed by age (β = 0.188), E/Ei ratio (β = 0.139) and Ei (β = 0.104), were the best correlates of LAVI (p at least < 0.01 for all) ().
Table IV. Clinical and echocardiographic correlates of left atrial volume index in the whole study population (n = 1104).
Discussion
The present study is the first one describing LA size and its biological covariates including tissue Doppler parameters in a large cohort of subjects with preserved systolic function examined in the setting of echocardiographic practice. Unlike previous reports carried out in population-based and selected research cohorts with prevalent cardiac disease, cardiac parameters were determined in individuals free of overt cardiovascular disease observed in current practice.
Our findings show that: (i) LAE is a frequent cardiac phenotype in the whole population peaking up to 20% in the elderly group; (ii) increased LV mass and LV diastolic dysfunction, as assessed by tissue Doppler indexes, emerge as major correlates of LAE independently of confounders.
Several aspects of our work deserve to be highlighted.
LAE may be misclassified by a single measurement of LAD, as the enlargement may result in asymmetrical LA geometry. This methodological aspect has limited the clinical value of LA size as a marker of cardiovascular risk. Despite this evidence and the growing interest in LA size as a marker of cardiovascular risk, a limited number of studies have evaluated LAV. Pritchett and coworkers (Citation21) assessed the prevalence of LAE and the association between LA parameters and cardiovascular disease; they were able to demonstrate that LAV indexed to body surface area was more strongly associated with cardiovascular diseases than LAD indexed to same index of body size, after adjusting for age and gender.
In a random-sample of 2042 residents of Olmsted County, Minnesota (USA), aged 45 years or more, LAE prevalence, identified according to partition values of LAV indexed to body surface area derived from a healthy reference group, was 16% (Citation21). In the MONIKA/KORA study, a population-based survey including 1212 individuals aged 25–74 years, LAV indexed to height was found enlarged in 9.6% men and 10% women (Citation4); these prevalence rates are similar to those found in the present study.
Prevalence of cardiac phenotypes, including LAE, may differ according to demographic and clinical characteristics of the study sample as well as the cut-off criteria used. At difference from Pritchett's study, our study design excluded subjects with impaired LV systolic function, atrial fibrillation, significant valve disease and ischemic cardiac disease, all conditions strongly related to LAE.
When LAE was defined by a single linear dimension [LAD index (LADI) ≥24 mm/m2] (Citation17) or by a less conservative LAVI threshold (≥24 ml/m2) (Citation11) than the 29 ml/m2 recommended by ASE guidelines (Citation17), LAE prevalence in our series increased from 10% to 18% and 21%, respectively.
Tissue Doppler imaging of mitral annulus during diastole is an established method for assessing cardiac function: the velocity of the earliest diastolic movement may reflect the rate of myocardial relaxation independently of after-load, pre-load and heart rate values. The E/Ei ratio is regarded as a tool for assessing LV filling combining the role of transmitral driving pressures and myocardial relaxation properties.
In simultaneous Doppler–catheterization comparison studies, E/Ei ratio values >15 have been shown to better correlate to LV filling characteristics than other conventional Doppler measurements in patients with normal or mildly impaired LV systolic function, but not in patients with advanced systolic heart failure (Citation19,Citation22). Based on this cut-off value, we found that LV filling index was elevated in about 5% of our sample, peaking to 11% in the highest LAVI quartile and to 12% in the elderly subgroup.
Furthermore, an impaired LV relaxation, according to age-related partition values for early myocardial diastolic velocity, was found in about a half of the study population. Our findings, in keeping with previous evidence (Citation23,Citation24), suggest that: (i) diastolic dysfunction, when assessed by comprehensive Doppler techniques, is a frequent finding in patients referred for routine echocardiographic examination; (ii) impaired diastolic function is properly detected only by integrating conventional and tissue Doppler parameters.
A notable aspect of our study is the relationship of LAV with LVH and diastolic dysfunction. A progressive increase in LV mass and a three-fold increase in the prevalence of LVH was found from the lowest to the highest LAVI quartile. This was also the case for abnormal indexes of LV relaxation and filling. The strength of such an association was supported by a multiple regression analysis showing that both LV mass index and tissue Doppler diastolic parameters were the most important correlates of LAVI.
It should be pointed out that LAE prevalence in current echocardiographic practice was probably substantially underestimated by the present study, which excluded all patients with a history of ischemic heart disease, atrial fibrillation, mitral annular calcification, prosthetic valve, moderate/severe mitral or aortic insufficiency, and depressed LV systolic function, in order to eliminate factors impacting on the accuracy of LV mass and diastolic indexes estimates.
Among the limitations of the study, we should mention that we did not have access to some clinical data including metabolic and renal parameters, which have been reported to correlate with LAVI and diastolic dysfunction (Citation3,Citation25). Finally, our observations cannot be generalized, since they refer to the setting of patients referred to echocardiography for routine clinical indications on the basis of the physicians’ clinical judgment.
In conclusion, LAE, as estimated from echocardiographic measurements of LAV rather than a single LAD (Citation26), was as high as 10% in a sample of patients free of overt cardiac disease. Higher LV mass, age and impaired LV diastolic function were significantly associated with a higher prevalence of LAE. Taken together, our findings reinforce the view that LA volume should be routinely estimated in echocardiography practice in order to identify patients at higher risk of heart failure and atrial fibrillation and to promote adequate prevention strategies.
Conflict of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Douglas PS. The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;44:1206–1207.
- Dwivedi G, Lip GY. The left atrium and diastolic dysfunction in hypertensive left ventricular hypertrophy: A consideration of size and function? J Hypertens. 2008;26: 1310–1312.
- Cuspidi C, Meani S, Fusi V, Valerio C, Catini E, Sampieri L, . Prevalence and correlates of left atrial enlargement in essential hypertension: Role of ventricular geometry and metabolic syndrome. J Hypertens. 2005;23:875–882.
- Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Doring A, . The aging process of the heart: Obesity is the main risk factor for the left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009;17:1982–1989.
- Oliveira W, Campos O, Bezerra Lira-Filho E, Cintra FD, Vieira M, . Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:1355–1361.
- Milan A, Caserta MA, Dematteis A, Naso D, Pertusio A, Magnino C, . Blood pressure levels, left ventricular mass and function are correlated with left atrial volume in mild to moderate hypertensive patients. J Hum Hypertens. 2009;23: 743–750.
- Toh N, Kanzaki H, Nakatani S, Ohara T, Kim J, Kusano KF, . Left atrial volume combined with atrial pump function identifies hypertensive patients with a history of paroxysmal atrial fibrillation. Hypertension. 2010;55:1150–1156.
- Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, . Cardiovascular features of heart failure with preserved ejection fraction versus non failing hypertensive left ventricular hypertrophy in the urban Baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207.
- Moller JE, Hillis GS, On JK, Seward JB, Reeder GS, Wright RS, . Left atrial volume. A powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212.
- Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, . Left atrial diameter as an independent predictor of first cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am Heart J. 2006;151:412–8.
- Leung DY, Chi C, Allman C, Boyd D, Ng AC, Kadappu KK, . Prognostic implications of left atrial volume index in patients with sinus rhythm. Am J Cardiol. 2010;105:1635–1639.
- Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Kober L, . Left atrial remodeling in patients with myocardial infarction complicated by heart failure, ventricular dysfunction or both: The VALIANT Echo study. Eur Heart J. 2009;30: 56–65.
- Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C. Left atrial volume in end-stage renal disease: A prospective cohort study. J Hypertens. 2006;24:1173–1180.
- Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, . Left atrial size and risk of major cardiovascular events during antihypertensive treatment. Losartan Intervention for Endpoint Reduction in Hypertension Trial. Hypertension. 2007;49:311–316.
- Cuspidi C, Negri F, Lonati L, Muiesan ML, Capra A, Milan A . Prevalence and correlates of echocardiographic left atrial enlargement in hypertensive outpatients in clinical practice. Clin Exp Hypertens. 2011, early online 1–8.
- Devereux RB, Reickek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63
- Verdecchia P, Angeli F, Gattobigio R, Sardone M, Porcellati C. Asymptomatic left ventricular systolic dysfunction in essential hypertension: Prevalence, determinants, and prognostic value. Hypertension. 2005;45:412–418.
- Janardhan R, Daley WL, Naqvi TZ, Mulvagh SL, Aurigemma G, Zile M, . Rationale and design: The VALsartan In Diastolic Dysfunction (VALIDD) trial: Evolving the management of diastolic dysfunction in hypertension. Am Heart J. 2006;152:246–252.
- Ommen SR, Nishimurua RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102: 1788–1794.
- Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: A population-based study. J Am Coll Cardiol. 2003;41:1036–1043.
- Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70.
- Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burdern of systolic and diastolic ventricular dysfunction in the community. JAMA. 2003;289: 194–202.
- Zanchetti A, Cuspidi C, Comarella L, Agabiti-Rosei E, Ambrosioni E, Chiariello M, . Left ventricular diastolic dysfunction in elderly hypertensives: Results of the APROS-diadys study. J Hypertens. 2007;25:2158–2167.
- Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, . Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112.
- Moya-Mur JL, Garcia-Martin A, Garcia-Liedo A, Ruiz-Leria S, Jimenez-Nacher JJ, Megias-Sanz A, . Indexed left atrial volume is a more sensitive indicator of filling pressures and left hart function than is anteroposterior left atrial diameter. Echocardiography. 2010;9:1049–1055.