Abstract
Objectives. Hypertension is a significant contributor to cardiovascular disease in HIV-infected individuals. The purposes of this study were to assess the development of new-onset hypertension and the use of antihypertensive treatment and blood pressure (BP) control. Methods. In a longitudinal study of 434 HIV-infected individuals (43±11 years, 72% males, follow-up 3.4±0.8 years), standardized BP recordings were undertaken at three clinical visits both at baseline and at follow-up, and cardiovascular risk factors were monitored. Adjusted odds ratio (OR) for new-onset hypertension (systolic BP≥140 and/or diastolic BP≥90 mmHg or initiation of antihypertensive treatment) was calculated using multiple logistic regression analyses. Results. New-onset hypertension occurred with an incidence of 29.8 per 1000 person-years (95% CI 20.3–42.2). HIV duration (OR=1.10, 95% CI 1.01−1.20), mean BP (1.24, 95% CI 1.13−1.35) and abnormal urinary albumin excretion (OR=5.47, 95% CI 1.07−27.85) were independent predictors for new-onset hypertension after adjustment. Use of antihypertensive treatment increased threefold from 17% to 49% in hypertensive patients. Adequate BP control was obtained in 22% of patients on antihypertensive therapy. Conclusions. HIV duration predicted new-onset hypertension, which could suggest involvement of low-grade inflammation; this hypothesis needs to be further explored. Despite increased use of antihypertensive treatment, enhanced awareness and adequate treatment of hypertension are still warranted in HIV-infected individuals.
Introduction
The introduction of antiretroviral therapy (ART) has led to a substantial decrease in HIV-related mortality; however, this has been accompanied by an increased risk of cardiovascular events in HIV- infected individuals (Citation1–3), up to twofold of that seen in the general population (Citation1–3). This amplified risk of cardiovascular disease (CVD) and related mortality has been linked to exposure to ART (Citation4) resulting in dyslipidemia and insulin resistance (Citation5,Citation6), as well as to the HIV infection itself, possibly mediated through immune dysfunction and low-grade inflammation (Citation7–9). Smoking is highly prevalent among HIV-infected patients and may contribute to the augmented cardiovascular risk (Citation10). As in the general population, hypertension is a significant contributor to cardiovascular disease among HIV-infected individuals (Citation10,Citation11). We have previously reported a 36% prevalence of hypertension in HIV-infected Caucasians, no different from the general Norwegian population (Citation12). The prevalence of hypertension in different selected HIV-infected cohorts varies considerably, from 13% to 49% (Citation9,Citation12–17). Population differences and methodological issues are likely explanations for the discrepancies in prevalence.
Increased blood pressure and development of hypertension have been observed after initiation of combined ART in prospective studies, effects that could be attributed to increased BMI, lipid profile and immunological status (Citation18–20). However, that cumulative exposure to ART leads to development of hypertension could not be confirmed in the large prospective multinational D:A:D (the Data Collection on Adverse Effects of Anti-HIV Drugs) study (Citation21). In the Swiss HIV cohort study, the prevalence of hypertension was found to be stable over a 6-year period (Citation17). Apart from these studies, there is no data on the development of hypertension in unselected HIV cohorts.
Awareness of hypertension in HIV-infected individuals and the recognition of hypertension as a contributor to cardiovascular disease in this population have increased during recent years. This is important, as hypertension is an amendable factor that can be treated effectively and most likely lead to improved morbidity and mortality. Whether the increased recognition of hypertension in HIV-infected individuals has led to change in treatment attitude, e.g. increased use of antihypertensive treatment and improved BP control, is not known.
The aims of this longitudinal study were to assess the incidence of new-onset hypertension in HIV-infected individuals over time and to identify predictors of new-onset hypertension, and furthermore, to assess the use of antihypertensive treatment and BP control.
Materials and methods
Participants
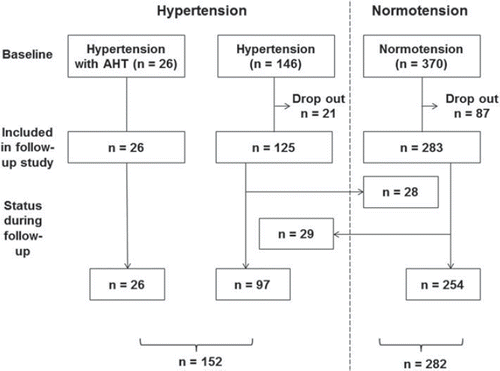
HIV-infected patients in the Oslo area are followed regularly at the Outpatient Infectious Disease Clinic at Oslo University Hospital, Ullevål. Participants in the initial “Microalbuminuria in the HIV-infected population of Oslo” (MAHO) study (Citation12) received a written invitation to participate in this preplanned follow-up study. Of the 542 patients who completed the baseline study, 434 patients were included in the follow-up study (). Patients were included between January 2007 and March 2009, and the database was closed February 2010. A total of 415 patients completed all three visits, eight patients attended two visits and 11 patients attended only one visit for various reasons (death, n=4; transfer to other hospitals, n=5; failure to show-up, n=10). Available data from all 434 patients were included in the final analyses. A total of 108 patients from the baseline study were lost to follow-up (death, n=15; transfer to other hospitals, n=10; lack of consent, n=83). These patients had lower cholesterol (4.8±1.1 vs 5.1±1.2 mmol/l, p=0.02), lower BP (126.6±16.1/78.4±9.5 vs 131.1±18.1/81.6± 10.9 mmHg, p=0.01/0.002) and were more likely to be normotensive (80.6% vs 65.2%, p=0.002) compared with the 434 that attended the follow-up study. The study was approved by the South-Eastern Regional Committee for Medical and Health Research Ethics, and concession was obtained from the National Data Inspectorate.
BP measurements and questionnaire
At the clinical visits, three at baseline and three at follow-up, BP measurements were performed in duplicate 2 min apart by well-trained nurses using a semiautomatic oscillometric device (Omron M4, Matsusaka Co. Ltd, Matsusaka, Japan). BP was measured in a quiet room in a sitting position after 5 min of rest and with an appropriate cuff size according to the upper arm circumference. The average of six systolic (S) and diastolic (D) BPs was used for statistical analysis both at baseline and at follow-up. In hypertensive patients who initiated antihypertensive drugs during the time of follow-up, the average of SBP and DBP measured after initiation of therapy was used. Mean BP was calculated as DBP + 1/3 (SBP-DBP). Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg or use of antihypertensive medication. New-onset hypertension was defined as normotension at baseline and hypertension at follow-up. Sustained hypertension was defined as hypertension at both baseline and follow-up. Adequate BP control was defined as SBP <140 mmHg and DBP <90 mmHg. BP level was classified according to the European Society of Hypertension (ESH)/European Society of Cardiology (ESC) 2007 guidelines with optimum/normal BP as SBP <130 and DBP <85 mmHg, high normal BP as SBP 130–139 mmHg and/or DBP 85–89 mmHg, grade I hypertension as SBP 140–159 mmHg and/or DBP 90–99 mmHg, grade II hypertension as SBP 160–179 mmHg and/or DBP 100–109 mmHg, grade III hypertension as SBP ≥180 mmHg and/or DBP ≥110 mmHg, and isolated systolic hypertension as SBP ≥140 mmHg and DBP <90 mmHg. If SBP and DBP were in different categories, the higher category was used (Citation22).
Demographics, laboratory data and estimation of cardiovascular risk
Information was collected with regard to current smoking, cardiovascular disease and use of antihypertensive drugs. Data on diabetes mellitus was obtained from the hospital's HIV database and the diagnosis confirmed from hospital records. Body weight was measured at the first visit. Demographic, clinical and laboratory data were obtained from the hospital's HIV database as described previously (Citation12). HIV RNA in EDTA plasma was quantified using polymerase chain reaction amplification with a COBAS Amplicor HIV-1 Monitor Test (Roche Diagnostics, Branchburg, NJ, USA). CD4 cell count was determined by routine flow cytometry using TriTest CD4/CD8 with TruCount Tubes (Becton Dickinson Biosciences, San Jose, CA, USA). All laboratory analyses were performed at the Department of Clinical Chemistry at Oslo University Hospital, Ullevål.
Urinary albumin excretion rate was measured as albumin-to-creatinine ratio (ACR) in urine at each clinical visit. If pyuria was present, the sample was discarded for further analysis. The average of three measurements was used for statistical analyses. Abnormal urinary albumin excretion was defined as microalbuminuria (i.e. ACR ≥2.5−30 mg/mmol in at least two of three samples) or ACR >30 mg/mmol but less than 150 mg/mmol, i.e. low-grade proteinuria.
HIV duration was estimated as the time period from the first HIV-positive test at the time of inclusion. Duration of ART was calculated as the cumulative exposure to a triple class regimen, and duration of protease inhibitor (PI) exposure was calculated separately as well. Current ART was defined as ART exposure at the time of inclusion, whereas those never exposed to ART were classified as ART-naïve. In statistical analyses of duration of ART, the ART-naïve patients were included and designated duration of 0 years. Glomerular filtration rate (GFR) was calculated according to the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula; eGFR (Citation23).
Ten-year risk of fatal cardiovascular disease was estimated according to the European cardiovascular disease risk assessment model, Systematic Coronary Risk Evaluation (SCORE) (Citation24). As Norway is categorized as a high-risk region, the SCORE high-risk chart was used, and the risk was estimated based on age, gender, SBP, cholesterol and smoking status (Citation24). Furthermore, stratification of cardiovascular risk into four categories was performed in the hypertensive patients according to the ESH/ESC 2007 guidelines (Citation22). Stratification was performed based on grade of hypertension together with the number of risk factors (men >55 years/women >65 years, smoking, cholesterol >5.0 mmol/l), presence of subclinical organ damage (eGFR <60 ml/min, abnormal albumin excretion) or disease (diabetes, CVD) at follow-up.
Statistical analyses
Data was collected using EpiInfo database (EpiInfoTM) and SPSS software was used for statistical analyses (SPSS Inc., PASW Statistics 18). Data are presented as mean ± SD, number or percentage, or if skewed, as median and 25th and 75th percentiles (interquartile range, IQR). For within-group comparisons, a paired t-test or Wilcoxon signed-rank test were used as appropriate, whereas binary data were tested using McNemar's test. For between-group comparisons, either a t-test or the Mann–Whitney was used. Dichotomous variables were compared using Fisher's exact test. Two multiple logistic regression analyses were undertaken. The first model explored predictors of new-onset hypertension among the normotensives at baseline, and the second model explored predictors of sustained hypertension among the hypertensives at baseline. New-onset hypertension or sustained hypertension was entered as the dependent variables, and putative independent categorical or continuous variables were selected on the basis of clinical knowledge and/or significant associations with outcome in univariate logistic analyses (p <0.1). As SBP and DBP were intercorrelated, mean BP was calculated and entered into the regression models. The number of independent variables was restricted due to the limited number of patients with the defined outcomes. The significance level was chosen as p<0.05.
Results
Population characteristics
The follow-up time was 3.4 ± 0.8 (range 1.7 − 5.7) years. Demographic and clinical characteristics of the study population (n = 434) are given in . The majority of the participants were male, and they were older than the females (45.4 ± 10.4 vs 36.9 ± 8.2 years, p < 0.001). Men were more often of Caucasian ethnicity (83.4% vs 40.5%, p < 0.001) and most of the non-Caucasian women came from HIV-endemic countries. Among the males, homosexual transmission was the most likely cause of HIV infection (65.8%).
Table I. Demographic, clinical, and laboratory data in 434 HIV-infected individuals at baseline and follow-up.
The prevalence of diabetes mellitus increased significantly during the follow-up period, while eGFR decreased (). More individuals had eGFR < 90 ml/min (11.8–18.2%, p < 0.001), while the proportion with eGFR < 60 ml/min remained unchanged during follow-up (1.2–2.1%, p = 0.13). The prevalence of obesity (i.e. BMI >30 kg/m2) was stable at 5%. Cholesterol level was unchanged as was the proportion with cholesterol ≥ 6.2 mmol/l (16.4% vs 17.1%, p = 0.82). ACR increased significantly albeit within the reference level, while the prevalence of patients with abnormal urinary albumin excretion remained unchanged (). The median CD4 cell count increased [380 (IQR 250–570) to 410 (290–560) cells/µl, p < 0.002], and fewer patients were ART-naïve during follow-up (). The SCORE 10-year risk estimate of fatal CVD increased during the time period (), while the proportion with CVD risk ≥ 5% at follow-up remained unchanged (8.3–9.2%, p = 0.48).
Hypertension status during follow-up
During follow-up, 10.2% (n = 29) developed new-onset hypertension (), corresponding to an incidence of 29.8 per 1000 person-years (95% CI 20.3–42.2), i.e. 3.1 ± 0.8% per year. Isolated systolic hypertension was observed in 13.4% of the patients who developed hypertension.
Compared with those who remained normotensive, patients with new-onset hypertension had more often BMI > 25 kg/m2, higher BP, higher ACR, and longer duration of both HIV and of ART at baseline (). In multivariate logistic regression analyses, baseline mean BP (OR = 1.24, 95% CI 1.13 − 1.35, p < 0.001), abnormal urinary albumin excretion (OR = 5.47, 1.07 − 27.85, p = 0.04) and HIV duration (OR = 1.10, 1.01 − 1.20, p = 0.02) were significant predictors of new-onset hypertension after adjustment for age, gender and BMI > 25 kg/m2. When entering duration of ART as an independent variable instead of HIV duration, duration of ART reached borderline statistical significance (OR = 1.17, 1.00–1.37, p = 0.053).
Table II. Clinical baseline characteristics of HIV-infected individuals (n= 283) who remained normotensive or became hypertensive at follow-up.
The prevalence of hypertension did not differ in the HIV cohort during follow-up (). Twenty-eight of the hypertensive patients at baseline became normotensive without use of antihypertensive drugs () and with significant decrease in BP [∆BP– 10.7 (IQR − 17.4 to − 7.6)/ − 4.5 (− 7.0 to − 0.7) mmHg, p < 0.001/ < 0.001].
Patients with sustained hypertension (n = 123) had higher mean BP (112.9 ± 9.6 vs 105.2 ± 4.6 mmHg, p < 0.001), less frequently isolated systolic hypertension (25.2% vs 67.9%, p < 0.001), and higher proportion with CD4 cell count ≥ 200 cells/µl at baseline (92.7% vs 78.6%, p = 0.04) compared with those who became normotensive. In a separate multivariate logistic regression model including only hypertensive patients at baseline (n = 151), mean BP (OR = 1.11, 95% CI 1.02 − 1.20, p = 0.01) and isolated systolic hypertension (OR = 0.31, 0.11–0.84, p = 0.02) were independent predictors of sustained hypertension after adjusting for age, BMI and CD4 cell count.
Antihypertensive treatment and BP control
Use of antihypertensive treatment increased threefold from 17.2% at baseline to 48.6% during follow-up. Of the 152 hypertensive patients, 78 did not use antihypertensive drugs, including 24 patients with new-onset hypertension. BP at follow-up did not differ between the hypertensive patients with and without antihypertensive treatment (). Grade III hypertension was present only in patients with antihypertensive therapy; otherwise the groups differed in age, eGFR and ACR as detailed in . More patients using antihypertensive treatment had a risk estimate of fatal CVD ≥ 5% (27.0% vs 14.1%, p = 0.05), and more had high or very high added cardiovascular risk compared with patients without antihypertensive treatment (58.1% vs 32.1%, p = 0.001).
Table III. Characteristics of HIV-infected hypertensive individuals with and without antihypertensive treatment at follow-up.
Monotherapy was used by 63.5% of the patients. Angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin II receptor blockers (ARB) were the most commonly used drugs whether used in monotherapy (57%) or in combined therapy (74%). Adequate BP control was obtained in 21.6% of all patients with antihypertensive treatment at follow-up; 30.8% of those with persistent antihypertensive treatment throughout the study and 16.7% of those starting antihypertensive treatment during follow-up (p = 0.16).
Discussion
An important clinical observation in this study was the threefold increase in use of antihypertensive drugs in HIV-infected hypertensive individuals during follow-up. This may reflect increased awareness of hypertension, possibly enforced by this ongoing study. Despite this encouraging observation, one half of the hypertensive individuals remained without antihypertensive treatment, and adequate BP control was achieved in only one fifth, slightly lower than what has been obtained in patients with essential hypertension in general practice in Norway (26%) (Citation25) and in Europe (28%) (Citation26). The prevalence of diabetes was nearly doubled, and the proportion of smokers remained unchanged during the study period; both worrisome influences with regard to cardiovascular risk.
New-onset hypertension was not as frequent in our study as observed in the D:A:D study (Citation21). In this study, 9000 normotensive HIV-infected patients, most living in European countries, were followed for approximately 2 years. The authors reported an incidence of new-onset hypertension of 72.1 per 1000 person-years; corresponding to an incidence of about 6.3% per year, around twofold of what we found and what has been observed in HIV-uninfected cohorts in Denmark (2.7% annual incidence) (Citation27), Italy (2.5%) (Citation28), Belgium (3.8%) (Citation29) and white populations in the USA (3.5–4.0%) (Citation30,Citation31). BP measurements in the D:A:D study were not performed in a standardized manner, and this could contribute to the disparate findings regarding incidence.
The duration of HIV infection was associated with development of hypertension in our study. That HIV infection per se could cause hypertension or BP elevation is not a novel assumption (Citation12,Citation13,Citation15, Citation19,Citation32,Citation33), and we have previously observed that HIV duration predicted ambulatory BP and ambulatory hypertension in patients with high casual BP (Citation34). Low-grade inflammation and immune activation persist in HIV-infected individuals despite antiretroviral therapy (Citation35,Citation36), and immune activation and inflammation could contribute to elevated BP (Citation37,Citation38).
Cumulative exposure to ART was related to new-onset hypertension. This is in contrast to the finding that cumulative exposure to non-nucleoside reverse transcriptase inhibitors was associated with a lower risk of new-onset hypertension in the D:A:D study (Citation21). However, initiation of ART has been related to elevated BP and development of hypertension in several studies (Citation16,Citation18–20), and exposure to ART has been linked to increased prevalence of hypertension in several cross-sectional and retrospective studies (Citation12,Citation16,Citation39), but results are conflicting (Citation13,Citation40). The mechanisms of the effect of ART on BP are not clear, but adverse metabolic effects of ART (Citation13,Citation14,Citation18,Citation19) as well as immune reconstitution in patients initiating ART (Citation18–20) could be important.
No change in the prevalence of hypertension was observed during follow-up, despite considerable dynamics in hypertension status. One fifth of the hypertensives became normotensive during follow-up, in keeping with what has been reported by others in HIV-uninfected populations (Citation29,Citation30). White coat hypertension and adaptation to BP measurements could possibly contribute to the transition from hypertension to normotension, compliant with our observation of isolated systolic hypertension as a negative predictor of sustained hypertension. We have previously reported that 25% of HIV-infected hypertensives have white coat hypertension (Citation34).
The most frequently used antihypertensive drugs were blockers of the renin–angiotensin system. This is in keeping with the recent guidelines of the European AIDS Clinical Society (EACS) on non-infectious co-morbidities in HIV (Citation41). Blockade of the renin–angiotensin system could be supported from the finding of Boccara et al. (Citation42) who observed that certain types of PI seem to be linked to activation of the renin–angiotensin system in cultured adipocytes. Blockade of the renin–angiotensin system is the primary therapeutic option in patients with early kidney damage, and our earlier finding of a threefold increased prevalence of microalbuminuria in HIV-infected persons compared with the general population (Citation41) lends support to the extensive use of ACE-I or ARB. Be that as it may, with the poor BP control observed in our population, the use of monotherapy is clearly too high, and more patients need combined antihypertensive therapy with an intensified BP lowering effect to obtain adequate BP control.
Our study has several strengths. Patients were recruited from an unselected HIV cohort from a single center, and the cohort was followed prospectively and according to a preplanned protocol. Standardized BP measurements were performed repeatedly and a total of six measurements at three visits were used to ensure proper classification of hypertension status at baseline as well as follow-up. Still, triplicate BP measurements at each visit would have been optimal. Furthermore, as white coat hypertension and masked hypertension can only be verified by ambulatory BP monitoring, lack of ambulatory BP measurement is a limitation of our study. The proportion of patients lost to follow-up, the limited number of patients, the observational design and the open nature of the study are additional shortcomings together with the short follow-up time and the lack of inflammatory biomarkers. Our findings in this study would need to be confirmed by long-term follow-up.
Conclusions
New-onset hypertension occurred at approximately the same rate as that observed in HIV-uninfected populations, but with considerably lower rate than previously reported from a large population of HIV-infected individuals. New-onset hypertension was independently predicted by HIV duration, suggesting that HIV-specific parameters such as immune activation and low-grade inflammation could be of importance in addition to traditional risk factors. A longer follow-up period, analyses of inflammatory biomarkers and an HIV-uninfected control group would be needed to explore possible HIV-specific contributions to new-onset hypertension. Antihypertensive drug treatment increased substantially during the time of follow-up period, indicating enhanced awareness of BP elevation in this patient population. As cardiovascular disease has increased in HIV-infected patients, high awareness and adequate treatment of hypertension is crucial to prevent subsequent hypertension-related disease in HIV-infected patients.
Acknowledgements
Presented in part: 21st European Meeting on Hypertension and Cardiovascular Prevention, Milan, June 17–20, 2011 (Abstract no. 462).
We acknowledge the skilled assistance of the nurses Astrid Marie Rudi, Kjersti Selnes, Lise Sørsvang, Jorun Almark and Linda Gail Skeie together with biomedical laboratory scientist Heidi Bertheussen at the outpatient clinic at the Department of Infectious Diseases. We also acknowledge the work of Professor Emeritus Johan N. Bruun in establishing the HIV cohort database. Professor Leiv Sandvik, Unit of Epidemiology and Biostatistics at Oslo University Hospital, Ullevål has given priceless advice on the statistics.
Sources of funding
The HIV fund of the Department of Infectious Diseases at Oslo University Hospital, Ullevål; South-Eastern Norway Regional Health Authority.
Conflicts of interest: None declared.
References
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512.
- Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, . Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512.
- Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, . Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin Infect Dis. 2007;44:1625–1631.
- Friis-Moller N, Sabin CA, Weber R, d’Arminio MA, El-Sadr WM, Reiss P, . Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349: 1993–2003.
- Riddler SA, Li X, Chu H, Kingsley LA, Dobs A, Evans R, . Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med. 2007;8:280–287.
- Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: A 5-year cohort study. Arch Intern Med. 2000;160:2050–2056.
- Kuller LH, Tracy R, Belloso W, De WS, Drummond F, Lane HC, . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5: e203.
- Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273.
- Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, . Low CD4 + T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447.
- Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, . Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356: 1723–1735.
- Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, . Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–270.
- Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: Influence of combination antiretroviral therapy. J Hypertens. 2008;26:2126–2133.
- Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naive and HIV-negative controls: Results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22:731–736.
- Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, . Hypertension among HIV patients: Prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382.
- Jerico C, Knobel H, Montero M, Sorli ML, Guelar A, Gimeno JL, . Hypertension in HIV-infected patients: Prevalence and related factors. Am J Hypertens. 2005;18: 1396–1401.
- Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, . Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960.
- Glass TR, Ungsedhapand C, Wolbers M, Weber R, Vernazza PL, Rickenbach M, . Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: The Swiss HIV Cohort Study. HIV Med. 2006;7:404–410.
- Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019–1026.
- Palacios R, Santos J, Garcia A, Castells E, Gonzalez M, Ruiz J, . Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Medicine. 2006;7:10–15.
- Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C. Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2003;4:411–416.
- Thiebaut R, El-Sadr WM, Friis-Moller N, Rickenbach M, Reiss P, Monforte AD, . Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10:811–823.
- Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, . European guidelines on cardiovascular disease prevention in clinical practice: Full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14 Suppl 2:S1–S113.
- Westheim A, Klemetsrud T, Tretli S, Stokke HP, Olsen H. Blood pressure levels in treated hypertensive patients in general practice in Norway. Blood Press. 2001;10:37–42.
- Kjeldsen SE, Naditch-Brule L, Perlini S, Zidek W, Farsang C. Increased prevalence of metabolic syndrome in uncontrolled hypertension across Europe: The Global Cardiometabolic Risk Profile in Patients with hypertension disease survey. J Hypertens. 2008;26:2064–2070.
- Asferg C, Mogelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, . Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. Am J Hypertens. 2010;23:327–333.
- Bombelli M, Facchetti R, Sega R, Carugo S, Fodri D, Brambilla G, . Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension. 2011.
- Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, . Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305: 1777–1785.
- Rywik SL, Williams OD, Pajak A, Broda G, Davis CE, Kawalec E, . Incidence and correlates of hypertension in the Atherosclerosis Risk in Communities (ARIC) study and the Monitoring Trends and Determinants of Cardiovascular Disease (POL-MONICA) project. J Hypertens. 2000;18: 999–1006.
- Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: A cohort study. Lancet. 2001;358:1682–166.
- Jung O, Bickel M, Ditting T, Rickerts V, Welk T, Helm EB, . Hypertension in HIV-1-infected patients and its impact on renal and cardiovascular integrity. Nephrol Dial Transplant. 2004;19:2250–2258.
- Medina-Torne S, Ganesan A, Barahona I, Crum-Cianflone NF. Hypertension is common among HIV-infected persons, but not associated with HAART. J Int Assoc Physicians AIDS Care (Chic ). 2011.
- Manner IW, Baekken M, Oektedalen O, Sandvik L, Os I. Effect of HIV duration on ambulatory blood pressure in HIV-infected individuals with high office blood pressure. Blood Press. 2010;19:188–195.
- Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, . T cell activation is associated with lower CD4 + T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543.
- Neuhaus J, Jacobs DR, Jr., Baker JV, Calmy A, Duprez D, La RA, . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795.
- Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951.
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, . Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140.
- Cattelan AM, Trevenzoli M, Sasset L, Rinaldi L, Balasso V, Cadrobbi P. Indinavir and systemic hypertension. AIDS. 2001;15:805–807.
- Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio MA, . Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193.
- EACS gulidelines, version 6.0. Web. 2011 Available from: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-6.pdf
- Boccara F, Auclair M, Cohen A, Lefevre C, Prot M, Bastard JP, . HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2010;15: 363–375.