Abstract
Background. Despite the proposal of different means of non-invasive arterial stiffness assessment, none offers simultaneous information on whole-body peripheral arterial condition. We investigated the validity of applying a six-channel electrocardiogram-based pulse wave velocity (ECG-PWV) measurement system for this purpose. Methods. The study consisted of two parts. Part One enrolled hypertensive (Group 1, n = 32) and normal (Group 2, n = 32) subjects, whereas Part Two recruited diabetic (Group 3, n = 50) and normal (Group 4, n = 50) subjects. To validate the application of ECG-PWV in assessing peripheral arterial stiffness in different parts of body, ECG-PWV data were compared with three other parameters including the cardio-ankle vascular index (CAVI), pulse wave velocity–digital volume pulse (PWV-DVP) and intima-media thickness (IMT). Results. ECG-PWV in healthy subjects in Part One correlated significantly with CAVI and PWV-DVP (p < 0.05), whereas ECG-PWV and CAVI were significantly different between the hypertensive and normal subjects. Moreover, comparison of IMT and ECG-PWV from different sites showed significant correlation only between IMT and ECG-PWV from earlobe (r = 0.495, p = 0.004). No significant association, however, was noted between IMT and CAVI. For Part Two, significant differences existed between diabetic and normal subjects in body weight, waist circumference, level of HbA1c, fasting blood sugar, serum creatinine and ECG-PWV from the foot. However, no significant difference was noted in PWV-DVP between two groups. Conclusions. Six-channel ECG-PWV measurement system showed remarkable correlation with IMT in hypertensive subjects and with key anthropometric and biochemical parameters in diabetic patients, suggesting its validity in assessing whole-body arterial stiffness in subjects with peripheral arterial diseases within 10 min.
Introduction
Among all the reported non-invasive tools in evaluation of the degree of atherosclerosis, assessment of arterial stiffness through pulse wave velocity (PWV) has previously been demonstrated to be an independent predictor of all-cause and cardiovascular mortality in patients with essential hypertension (Citation1–3). Although conventional PWV is widely used clinically for early detection of vascular pathology, it focuses on the assessment of atherosclerotic change of the aorta (Citation4–10). Since atherosclerosis of peripheral artery may be an earlier presentation of the systemic disease, PWV–digital volume pulse (DVP) was introduced in 2005 to evaluate the arterial stiffness of peripheral arteries through analyzing the difference in transmission time between the finger and toe (Citation11). In addition to PWV (Citation5–8,Citation11), another PWV-based assessment tool commonly used at medical institutions is the cardio-ankle vascular index (CAVI) that evaluates the degree of atherosclerosis from data obtained through the ankles and the arms (Citation12–15). However, both PWV-DVP and CAVI can only assess the degree of local atherosclerosis over the extremities, whereas similar data cannot be acquired from other parts of the body. Intima-media thickness (IMT), which is not a PWV-based parameter, is another widely accepted clinical index for evaluating the degree of atherosclerosis at a supra-clavicular level by using B-mode ultrasonography (Citation16–19). Although the degree of vascular obstruction can be directly visualized using this parameter, the field of detection is limited to part of the cervical region and the degree of atherosclerosis of the body as a whole cannot be evaluated. To assess the whole-body atherosclerotic status, Gladdish et al. (Citation20) attempted to determine the PWV over the brain, ear and finger using the non-invasive means of transcranial Doppler and plethysmography through simultaneous measurements from the temporal window, left middle finger, and right ear lobe of 11 healthy volunteers. The clinical application of this parameter, however, is hampered by the need for expensive equipment and trained personnel.
To achieve the same purpose of simultaneous whole-body assessment, we have previously proposed a six-channel electrocardiography (ECG)–PWV measurement instrument that can complete the calculations of six PWV values obtained from bilateral earlobes, fingers and toes within 10 min. The data thus obtained were close to those previously reported by others (Citation20). Although the reliability of its use in normal testing subjects has been validated in our previous study (Citation21), it has not been tested in subjects with known cardiovascular disease (CVD). The aims of this study are to validate the application of this parameter in assessing the degree of atherosclerosis in the diseased population and to compare the values obtained with those of IMT, CAVI and PWV-DVP. As hypertension and diabetes are the two most commonly encountered cardiovascular risk factors contributing to the development of peripheral arterial disease, patients belonging to these two categories were chosen for the present study (Citation22).
Despite its nature as a systemic disease, the degree of atherosclerosis may be different over various parts of the body. For instance, patients with stroke may have more advanced disease progression over the head and neck regions, whereas diabetic patients may sustain a more severe condition over the lower extremities. The present study attempted to validate the use of ECG-PWV in the evaluation of the degree of atherosclerosis on a whole-body basis within a short time (i.e. 10 min). In the first part of the study, the use of ECG-PWV in assessing the degree of arterial stiffness in hypertensive patients with or without history of stroke was described. The second part of the study, on the other hand, recruited patients with known diabetes. The applications of ECG-PWV indices obtained from peripheral arteries over different parts of the body were then evaluated, followed by discussion of our findings.
Patients and methods
Study population
To evaluate the validity of applying ECG-PWV in assessing peripheral arterial stiffness in different clinical settings of peripheral vascular disease, this study consisted of two parts. Part One of the study focused on the use of ECG-PWV in assessing the degree of arterial stiffness in hypertensive patients with or without history of stroke, whereas Part Two of the study recruited patients with known type 2 diabetes. Normal subjects were enrolled in both parts to serve as normal controls.
Part One enrolled 64 subjects from January 2008 to August 2009. Subjects were considered hypertensive if the systolic blood pressure was over 140 mmHg and/or a diastolic pressure above 90 mmHg, or if they were under anti-hypertensive treatment. Of all the subjects, 32 with a known history of hypertension for at least 6 months with or without a history of stroke were recruited from the outpatient clinic of Buddhist Tzu Chi General Hospital (Group 1). Subjects with a known history of other atherosclerosis-related complications, such as angina, myocardial infarction and intermittent claudication within 6 months were excluded from this study. Those with a known diagnosis of diabetes and/or renal diseases were also excluded. The other 32 subjects were healthy volunteers without known history of systemic disease including hypertension, diabetes mellitus, stroke or other cardiovascular diseases (Group 2).
Of the 32 subjects in Group 1, 20 were males. Fifteen subjects in Group 1 had concurrent hypertension and history of stroke, whereas the other 17 subjects had hypertension only. Of the 32 subjects in Group 2, 19 were males. The anthropometric data for Groups 1 and 2 are shown in .
Table I. Basic demographic, anthropometric and hemodynamic parameters of subjects in Part One.
PWV-related parameters, including CAVI, PWV-DVP and ECG-PWV, were obtained from all 64 subjects, whereas IMT measurement was performed only for those in Group 1. The study protocol was approved by the Institutional Review Board on Human Research at Buddhist Tzu Chi General Hospital and National Dong Hwa University. Informed consents were obtained from all subjects.
Part Two recruited diabetic patients from the diabetic outpatient clinic of the Hualien Hospital from July 2009 to August 2010. In tota, 50 patients with an established diagnosis of diabetes mellitus type 2 were enrolled (Group 3). There were 33 males. Diabetes was diagnosed by either a fasting sugar level higher than 126 mg/dl or a glycosylated hemoglobin (HbA1c) level > 6.5% (Citation24). They all received regular treatment and follow-up in the clinic for more than 2 years. The basic demographic and anthropometric characteristics of the study subjects are shown in . Another 50 healthy subjects were recruited from a health examination program at the same hospital to serve as normal controls (Group 4). There were 27 males in this group. The criteria for recruitment included an absence of history of diabetes mellitus, a fasting sugar level lower than 126 mg/dl and an HbA1c level less than 6.5%. Subjects with history of hypertension and/or atherosclerosis-related complications, such as angina, myocardial infarction, stroke and peripheral vascular diseases within 3 months were excluded from the Part Two. All subjects underwent ECG-PWV and PWV-DVP measurements. The study protocol was approved by the Institutional Review Board on Human Research at Hualien Hospital. Informed consents were obtained from all subjects.
Table II. Basic demographic, anthropometric, and hemodynamic data of diabetic (Group 3) and healthy (Group 4) subjects in Part Two (n = 100).
Protocol of measurement of six-channel ECG-PWV and other parameters
Before measurement was taken for both parts of the study, a questionnaire was given to each testing subject to obtain detailed information on general body condition and medical history. Age and gender as well as anthropometric data including body weight, body height and waist circumference were also recorded. All subjects were allowed to rest in a supine position in a quiet, temperature-controlled room at 26 ± 1°C for 5 min before another 5 min of measurement. The study parameters were slightly different between Parts One and Two. Whereas CAVI, PWV-DVP, ECG-PWV and IMT were utilized in Part One, only PWV-DVP and ECG-PWV were obtained from subjects participating in Part Two. The same personnel from the neurosonographic laboratory at Tzu Chi General Hospital, who were blinded to the study design, were responsible for operation of the same equipment in both Parts One and Two studies.
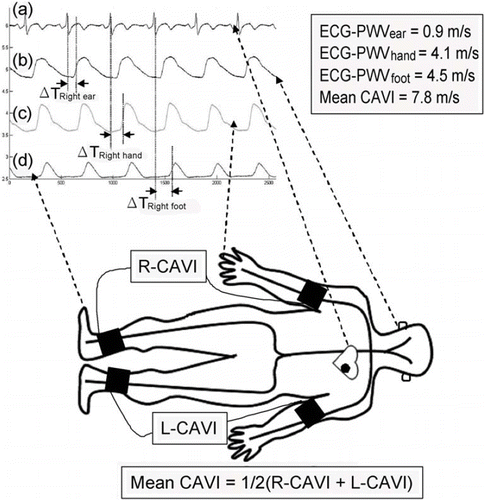
For ECG-PWV measurement, the distances from sternal notch to different points of reference were measured with the subjects in supine position and labeled as Li. The shortest distances from the sternal notch to the right and left ear lobes (i.e. LRight ear and LLeft ear, respectively) were measured with the subject's eyes staring at the ceiling. The shortest distances from sternal notch to the right and left index fingers (i.e. LRight hand and LLeft hand, respectively) were measured with the subjects’ arms stretched and abducted 90° from the body on a horizontal plane. On the other hand, the distance from the sternal notch to the foot (i.e. LRight foot and LLeft foot, for right and left foot, respectively) was the sum of the shortest distance from the sternal notch to medial patella, from medial patella to medial malleolus, and from medial malleolus to the tip of the second toe. Six infrared sensors were put on the points of reference simultaneously to acquire data on ECG-PWVs. ECG was obtained using the conventional method. Because of its conspicuousness, the R wave on Lead II was chosen as a reference point, the time from which to the foot point of a pulse wave was defined as the time difference (ΔTi) (Citation22) (). Hence, the mean ECG-PWVs from both sides of the earlobes (i.e. ECG-PWVear), hands (i.e. ECG-PWVhand) and feet (i.e. ECG-PWVfoot) can be determined by averaging the values of Li/ΔTi from both sides.
Figure 1. Schematic illustration of the measurements of six-channel electrocardiogram-based pulse wave velocity (ECG-PWV) and cardio-ankle vascular index (CAVI). With (a) R wave on Lead II as a reference point, the time differences (ΔTi) to (b) ear lobe, (c) index finger and (d) second toe were obtained. ECG-PWV was calculated by dividing the distances from different points of reference (Li) with ΔTi (i.e. ECG-PWVi = Li/ΔTi). Mean CAVI was obtained by averaging the values from both sides of the body as shown in the equation.
Signals of DVP were obtained by the aforementioned system (Citation25–27). Briefly, DVP signals of six-channel were recorded for a 300-s period. After being processed through an analog-to-digital converter (USB-6009 DAQ, National Instruments, TX, USA) with a sampling frequency of 200 Hz, the digitized signals were stored on a computer. Subsequent analyses included identification of foot points of DVPs, thereby obtaining individual waveforms. The ECG-PWVs from each point of reference within the 300 s of recording were averaged to yield six mean values from the six respective points of reference. The reproducibility of the results was confirmed following the protocol previously described (Citation22,Citation27).
PWV-DVP, which is an index of atherosclerosis over the extremities (Citation13), was defined as the difference between Lfoot and Lhand divided by the time difference between the foot points of the two DVPs (Δt) [i.e. (Lfoot − Lhand)/Δt], where Lfoot and Lhand are the mean of right and left sides for the upper and lower extremities, respectively.
CAVI, which is a phonocardiogram (PCG)-based index reflecting arterial stiffness from the heart to ankles known to increase with the degree of arteriosclerosis, was measured using the Vascular Screening System (VaSera VS-1000, Fukuda Denshi Co., Ltd., Tokyo, Japan). Two values of CAVI from both sides of the body can be obtained that have been demonstrated to show consistent correlations with IMT and PWV (Citation14,Citation15). The subjects were in supine position with pressure cuffs on both arms and lower legs. PCG sensor was placed at the right sternal border over the second intercostal space. Limb cushions were used to avoid limb contact with the bed, thereby minimizing potential variation. Right and left extremities were pressurized alternatively to generate pulse waves from which CAVI was calculated (i.e. R-CAVI and L-CAVI, respectively). The schematic presentation of CAVI and ECG-PWV measurement is shown in .
For IMT assessment, carotid scanning was performed with an ultrasonography machine (General Electric Logic 7; USA) equipped with a 10-MHz linear probe at B-mode (Citation25). IMT was measured 1 cm distal to the bulbus over a length of 1 cm for both carotid arteries. Measurements at three different locations were taken from right and left carotid arteries. The highest value was taken as the desired IMT (i.e. Max IMT) after data collection from both sides. An IMT over 0.822 mm has been reported in patients with cardiovascular diseases (Citation28). An increase in IMT has also been demonstrated in patients with known coronary artery disease (CAD) compared with those without (Citation29).
Statistical analysis
Associations between IMT and ECG-PWVs from different points of reference as well as correlations among ECG-PWVs from different regions in Group 1 subjects in Part One were evaluated by the Pearson correlation test. For comparisons between two groups in both parts of the study, an independent samples t-test was used. All data were expressed as mean ± standard deviation. Statistical analyses were performed by using SPSS software, Version 14.0 for Windows (SPSS Inc., Chicago, IL). A p-value < 0.05 was considered statistically significant.
Results
Agreement between data on ECG-PWV from both sides of the body
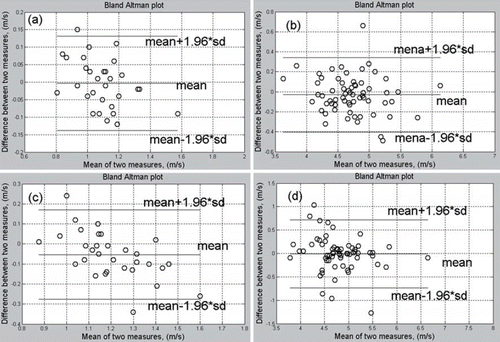
Significant agreement of data on ECG-PWV between both sides of the body from different reference points for measurement in healthy (Group 2, n = 32) and the hypertensive (Group 1, n = 32) subjects was noted using Bland–Altman analysis ().
Figure 2. Bland–Altman plot among data of six-channel electrocardiogram-based pulse wave velocity (ECG-PWV) from both sides of the body in healthy (Group 2, n = 32, a and b) and hypertensive (Group 2, n = 32, c and d) subjects, showing significant agreement between right and left sides with earlobes (a, c), and fingers and toe (b, d), being the points of reference for measurement.
Performance of ECG-PWV indices in hypertensive subjects (Part One)
shows that the values of ECG-PWV in healthy subjects enrolled in Part One (i.e. Group 2) correlated significantly with those of CAVI and PWV-DVP from different points of reference (p < 0.05). demonstrated that although no significant difference was noted in PWV-DVP between Group 1 and Group 2 subjects, the values of ECG-PWV and CAVI were significantly different between the two groups with the exception of the values of ECG-PWV from hand. Moreover, significant difference was also noted in the mean values of CAVI between Group 1 and Group 2. The former exhibited higher values and wider standard deviation albeit within normal range (i.e. < 9), whereas the latter showed significantly lower values with comparatively narrow standard deviations.
Table III. Associations of six-channel electrocardiogram-based pulse wave velocity (ECG-PWV) with cardio-ankle vascular index (CAVI) and pulse wave velocity–digital volume pulse (PWV-DVP) in healthy (Group 2) subjects.
Table IV. Comparisons among six-channel electrocardiogram-based pulse wave velocity (ECG-PWV), cardio-ankle vascular index (CAVI), maximum intima-media thickness (Max IMT) and pulse wave velocity–digital volume pulse (PWV-DVP) in hypertensive (Group 1) and healthy (Group 2) subjects.
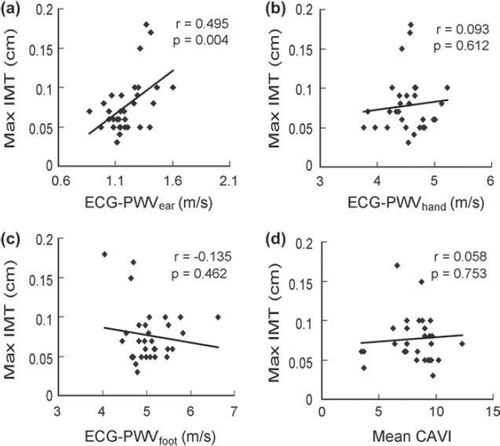
Values of Max IMT in Group 1 subjects exhibited no significant correlations with ECG-PWVhand (p = 0.612) (), ECG-PWVfoot (p = 0.462) () and CAVI (p = 0.753) (). Comparison of Max IMT and ECG-PWV from different sites of measurement showed that significant correlation existed only between Max IMT and ECG-PWVear (r = 0.495, p = 0.004) ().
Figure 3. Correlations of maximal intima-media thickness (Max IMT) with six-channel electrocardiogram-based pulse wave velocity (ECG-PWV) from different points of reference. Correlation of Max IMT with (A) ECG-PWVear, (B) ECG-PWVhand, (C) ECG-PWVfoot, and (D) mean cardio-ankle vascular index (CAVI) in hypertensive (Group 1) subjects (n = 32).
Performance of ECG-PWV indices in diabetic subjects (Part Two)
For Part Two, there were significant differences between diabetic (i.e. Group 3) and normal (i.e. Group 4) subjects in terms of body weight, waist circumference (), level of HbA1c, high- density lipoprotein, creatinine, cholesterol, triglyceride and fasting blood sugar as well as mean ECG-PWVfoot (). On the other hand, no significant difference was noted in PWV-DVP between the two groups.
Table V. Serum biochemical, and pulse wave velocity measurement parameters [i.e. 6-channel electrocardiogram-based pulse wave velocity (ECG-PWV) and pulse wave velocity–digital volume pulse (PWV-DVP)] in diabetic (Group 3) and healthy (Group 4) subjects (n = 100)].
Discussion
Compared with pulse pressure, which is an indirect index of arterial stiffening, PWV has been demonstrated to be an independent predictor of all-cause and cardiovascular mortality in patients with essential hypertension (Citation1). Measurement of the PWV has become one of the most common non-invasive parameter adopted in the assessment of the degree of atherosclerosis (Citation6,Citation7). A dual-channel instrument, PWV-DVP, has previously been introduced as a parameter for evaluating the degree of atherosclerosis over upper and lower extremities (Citation11). However, the analysis was performed only on 100 testing subjects without history of significant CVD. It has been reported previously that the degree of atherosclerosis from different parts of the body is the same for subjects without cardiovascular diseases (Citation21). Consistent with the finding of that study, the results of the current study showed that there was no significant difference in the accuracy of measurement on PWV using different systems (ECG-PWV, PWV-DVP and CAVI). Accordingly, Part One of the study demonstrated highly significant associations of ECG-PWV from different points of reference with CAVI and PWV-DVP (). In other words, positive correlation exists between the results of measurement in normal healthy subjects regardless of the method used (Citation21).
Since the measurement of PWV using currently available instruments is based on only two local points of reference with a wide range of variability in different regions of the body (Citation23,Citation29,Citation30), concomitant assessment of the degree of atherosclerosis of the whole body is not feasible. To tackle the problem, a previous study has proposed the use of electrocardiogram (ECG) as a time reference when measuring the pulse transmission time (PTT) between bilateral ear lobes, fingers and toes (Citation31). According to that study, PTT is defined as the duration between the R wave of ECG and the trough of the pulse wave. However, unlike PWV, PTT is a relative value that can be affected by individual characteristics (e.g. body height) and, therefore, may not accurately reflect the degree of atherosclerosis. To further refine the technique, we adopted six points of reference to obtain an absolute value of PWV (i.e. ECG-PWVi) by dividing the distance measured (i.e. Li) with the time elapsed (i.e. ΔTi) ().
Consistent with our previous finding that showed highly significant agreement between both sides of the body in healthy subjects (Citation21), the current study also demonstrated significant symmetry between both sides in the hypertensive participants using Bland–Altman analysis (). Based on this symmetry in ECG-PWV in the testing subjects, the mean values of ECG-PWVear, ECG-PWVhand and ECG-PWVfoot were adopted in the assessment of the degree of atherosclerosis over head-neck region, upper and lower extremities, respectively. In Part One of this study, three PWV-related systems were utilized (i.e. ECG-PWV, PWV-DVP and CAVI). In Part One, since Group 1 recruited aged and hypertensive patients and Group 2 enrolled young normotensive healthy volunteers, we proposed a more prominent atherosclerotic change in Group 1 subjects compared with those in Group 2. The results showed that PWV-DVP failed in detecting significant difference in the degree of atherosclerosis between the aged hypertensive (Group 1) and young normotensive (Group 2) subjects (). On the other hand, although CAVI demonstrated a significant difference between the two groups, it exhibited normal values (i.e. < 9) in the majority of hypertensive patients, even in nine participants with a previous history of stroke (CAVI = 8.43 ± 2.26, individual data not shown). The results, therefore, suggest that CAVI may underestimate the degree of vascular pathology in hypertensive subjects. Hence, of particular importance in the present study is that ECG-PWVear and ECG-PWVfoot successfully distinguished between Group 1 and Group 2 in terms of the degree of atherosclerosis (p = 0.022 and p = 0.009, respectively) ().
Unlike PWV-DVP and CAVI, IMT has been reported to be a reliable independent predictor of stroke (Citation32). On the other hand, another study on 20 healthy subjects comparing the correlations among brachial–ankle PWV, IMT and CAVI demonstrated no significant association both between IMT and brachial–ankle PWV as well as between IMT and CAVI (Citation24). Previous literature has systematically compared CAVI, IMT and PWV in terms of their accuracy and reliability in assessing the degree of atherosclerosis (Citation14,Citation24,Citation33). A significant correlation has been reported between CAVI and IMT in 1014 hypertensive subjects over the age of 40 without history of stroke and cerebrovascular diseases. The results of the present study showed that while PWV-DVP and CAVI exhibited no notable association with IMT, ECG-PWVear showed a significant positive correlation with IMT (r = 0.495, p = 0.004) (). This novel parameter therefore may serve as a reliable alternative to IMT in assessing the risk of atherosclerosis of carotid artery. Consistent with the findings of previous studies showing a lack of significant correlation between IMT and systemic risk factors of atherosclerosis (Citation34) as well as a dissociation between IMT and conventional PWV (Citation34,Citation35), our results further suggest that the progression of atherosclerosis may be different at various sites over the body. Our finding that only ECG-PWVear correlated with IMT reinforces the validity of the use of six-channel ECG-based PWV measurement in the region-by-region assessment of whole-body arterial stiffness. Another point of importance is that while PWV-DVP, CAVI and IMT can only assess the degree of local atherosclerosis, ECG-PWV can reliably reflect the degree of atherosclerotic change of the whole body because of simultaneous measurements from different points of reference over the head as well as the upper and lower extremities. For Part Two, there were significant differences between diabetic and normal subjects in terms of body weight, waist circumference, levels of HbA1c, fasting blood sugar, and serum creatinine as well as ECG-PWVfoot. On the other hand, no significant difference was noted in PWV-DVP between the two groups.
Unlike normal control subjects that showed significant correlations among ECG-PWVs from different points of reference, ECG-PWVear was significantly elevated in hypertensive subjects and did not correlate with ECG-PWVhand and ECG-PWVfoot. In addition, ECG-PWVear was significantly associated with IMT that reliably reflects the degree of atherosclerosis of carotid arteries. On the other hand, the present study showed that diabetic patients had significantly elevated body weight, waist circumference, level of HbA1c, fasting blood sugar and serum creatinine. These findings are consistent with those of previous studies (Citation28,Citation36). More importantly, although PWV-DVP failed in demonstrating a discrepancy between diabetic and normal subjects that is supposed to exist due to impaired circulation of the lower extremities in the diabetics (Citation37), significant difference in ECG-PWVfoot was noted between the two groups. The findings, therefore, demonstrated that the region at risk of atherosclerotic change may be assessed using ECG-PWV. This is particularly useful in locating vascular lesion for subjects at risk of atherosclerotic disease who receive no previous screening. Other advantages of adopting ECG-PWV in assessing arterial stiffness are that not only can six values of PWVs be obtained simultaneously within 10 min under the same physiological condition, but there is also no need for expensive accessories as in IMT measurement. Besides, physicians can precisely assess the location of anomalies in arterial stiffness according to the six values available for interpretation, thereby giving advice to subjects at risk for prevention or treatment of their atherosclerotic condition. Furthermore, since the normal range of PWV from each reference point is available, the subjects being tested can also keep record of their changes in values to track the progression of their peripheral arterial condition so that they can seek medical help when abnormalities arise. In this way, the ECG-PWV measurement system may serve as a user-friendly tool for both health professionals and population at risk in the early detection of peripheral arterial disease and also in following treatment outcome after implementation of different therapeutic measures.
There are, however, certain limitations in using photoplethysmography sensors for detecting DVP signal. Firstly, readings from infrared sensors may be influenced by skin pigmentation, individual tissue characteristics and initial blood flow in the measured area (Citation38). Secondly, the signals may also be affected by involuntary vibrations of the examinees. Thirdly, decreased signal amplitude may occur in subjects with impaired peripheral circulation. Fourthly, the measurement may also be affected by a decreased environmental temperature that tends to cause constriction of the peripheral vessels. The impact of the last condition can be minimized by elevating the room temperature at the time of measurement. Fifthly, particular attention has to be paid to the measurement of distance from sternal notch to the point of reference to minimize error in calculation of ECG-PWVs. Finally, since the examinees in Part One of the present study were not age-matched, the influences of age and hypertension cannot be differentiated. Nevertheless, since the primary aim of this study was not to differentiate between the two disease conditions but to validate the use of a new set of parameters to assess atherosclerotic change over different parts of the body, the significant difference in age between subjects in Group 1 and Group 2 had an unfavorable but relatively insignificant impact on our study.
In conclusion, the six-channel ECG-PWV measurement system showed remarkable correlation with IMT in hypertensive subjects and with key anthropometric and biochemical parameters in diabetic patients, suggesting its validity in assessing arterial stiffness in subjects with peripheral arterial diseases. Moreover, given its capability of acquiring simultaneous PWVs from the body as a whole within 10 min and the ease of operation, it may serve as a convenient tool for early detection and following the progression of atherosclerosis in the population at risk.
Acknowledgements
The authors would like to thank Miss Li-Sheng Kuo who provided professional technical support in sonographic study for IMT assessment at Buddhist Tzu Chi General Hospital. A tribute is also paid to the volunteers involved in this study for allowing us to collect and analyze their data. Moreover, the authors are grateful for the support of Texas Instruments, Taiwan, in sponsoring the low power instrumentation amplifiers. This research was partly supported by National Science Council under Grant NSC99 - 2221-E-259 - 001, Taiwan, Republic of China.
Conflict of interest: No conflict of interest to declare.
References
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Achimastos A, Benetos A, Stergiou G, Argyraki K, Karmaniolas K, Thomas F, . Determinants of arterial stiffness in Greek and French hypertensive men. Blood Press. 2002;11:218–222.
- Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro Moraes C, . Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press. 2012;21:31–38.
- Lim HE, Park CG, Shin SH, Ahn JC, Seo HS, Oh DJ. Aortic pulse wave velocity as an independent marker of coronary artery disease. Blood Press. 2004;13:369–375.
- Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension. 2009;54:1328–1336.
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117.
- Wilkinson IB, Webb DJ, Cockcroft JR. Aortic pulse-wave velocity. Lancet. 1999;354:1996–1997.
- Alty SR, Angarita-Jaimes N, Millasseau SC, Chowienczyk PJ. Predicting arterial stiffness from the digital volume pulse waveform. IEEE T Bio-Med Eng. 2007;54:2268–2275.
- Wakabayashi I, Masuda H. Association of pulse pressure with carotid atherosclerosis in patients with type 2 diabetes mellitus. Blood Press. 2007;16:56–62.
- Filipovsky J, Ticha M, Cifkova R, Lanska V, Stastna V, Roucka P. Large artery stiffness and pulse wave reflection: Results of a population-based study. Blood Press. 2005;14:45–52.
- Tsai WC, Chen JY, Wang MC, Wu HT, Chi CK, Chen YK, . Association of risk factors with increased pulse wave velocity detected by a novel method using dual-channel photoplethysmography. Am J Hypertens. 2005;18:1118–22.
- Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, . Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circulation. 2007;71: 1710–1714.
- Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101–107.
- Wakabayashi I, Masuda H. Association of acute-phase reactants with arterial stiffness in patients with type 2 diabetes mellitus. Clin Chim Acta. 2006;365:230–235.
- Shen T W, Wang CH, Lai YH, Hsu BG, Liou HH, Fang TC. Use of cardio-ankle vascular index in chronic dialysis patients. Eur J Clin Invest. 2011;41:45–51.
- Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, . The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta- analysis. Circulation. 2007;115:459–467.
- Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: A new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20: 159–169.
- Palatini P, Puato M, Rattazzi M, Pauletto P. Effect of regular physical activity on carotid intima-media thickness. Blood Press. 2010;20:37–44.
- Gladdish S, Manawadu D, Banya W, Cameron J, Bulpitt CJ, Rajkumar C. Repeatability of non-invasive measurement of intracerebral pulse wave velocity using transcranial Doppler. Clin Sci (Lond). 2005;108:433–439.
- Liu AB, Hsu PC, Chen ZL, Wu HT. Measuring pulse wave velocity using ECG and photoplethysmography. J Med Syst. 2011;35:771–777.
- Chi YW, Jaff MR. Peripheral artery disease and genetics: Is there a cause-and-effect relationship? Postgrad Med. 2010;122:170–176.
- Tillin T, Chambers J, Malik I, Coady E, Byrd S, Mayet J, . Measurement of pulse wave velocity: Site matters. J Hypertens. 2007;25:383–389.
- Huck CJ, Bronas UG, Williamson EB, Draheim CC, Duprez DA, Dengel DR. Noninvasive measurements of arterial stiffness: Repeatability and interrelationships with endothelial function and arterial morphology measures. Vasc Health Risk Manag. 2007;3:343–349.
- Wu HT, Liu CC, Lin PH, Chung HM, Liu MC, Yip HK, . Novel application of parameters in waveform contour analysis for assessing arterial stiffness in aged and atherosclerotic subjects. Atherosclerosis. 2010;213:173–177.
- Aminbakhsh A, Mancini GB. Carotid intima-media thickness measurements: What defines an abnormality? A systematic review. Clin Invest Med. 1999;22:149–157.
- Coskun U, Yildiz A, Esen OB, Baskurt M, Cakar MA, Kilickesmez KO, . Relationship between carotid intima-media thickness and coronary angiographic findings: A prospective study. Cardiovasc Ultrasound. 2009;7:59.
- Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85.
- McLeod AL, Uren NG, Wilkinson IB, Webb DJ, Maxwell SR, Northridge DB, . Non-invasive measures of pulse wave velocity correlate with coronary arterial plaque load in humans. J Hypertens. 2004;22:363–368.
- Yu WC, Chuang SY, Lin YP, Chen CH. Brachial–ankle vs carotid–femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31.
- Allen J, Murray A. Variability of photoplethysmography peripheral pulse measurements at the ears, thumbs and toes. IEE P-Sci Meas Tech. 2000;147:403–407.
- Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 2005;179:325–331.
- Kadota K, Takamura N, Aoyagi K, Yamasaki H, Usa T, Nakazato M, . Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circulation. 2008;72:304–308.
- Stompor T, Rajzer M, Pasowicz M, Krasniak A, Sulowicz W, Kawecka-Jaszcz K, . Coronary artery calcification, common carotid artery intima-media thickness and aortic pulse wave velocity in patients on peritoneal dialysis. Int J Artif Organs. 2006;29:736–744.
- Zureik M, Temmar M, Adamopoulos C, Bureau JM, Courbon D, Thomas F, . Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens. 2002;20:85–93.
- Lehto S, Niskanen L, Ronnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin- dependent diabetes mellitus. Stroke. 1998;29:635–639.
- Kizu A, Koyama H, Tanaka S, Maeno T, Komatsu M, Fukumoto S, . Arterial wall stiffness is associated with peripheral circulation in patients with type 2 diabetes. Atherosclerosis. 2003;170:87–91.
- Schultz EU, Blazek V. Value of quantitative photoplethysmography for functional vascular diagnostics – Current status and prospects. Skin Pharmacol Appl. 2001;14:316–323.