Abstract
Objective. Isolated systolic hypertension (ISH) is generally encountered in elderly patients and there are scarce data regarding the renin–angiotensin–aldosterone system (RAAS) activity in patients with ISH. We aimed to determine the plasma renin activity (PRA), plasma aldosterone levels (PAL) and aldosterone/PRA ratio (PAL/PRA) in patients (age >50 years) with ISH and to compare these values with patients with essential hypertension (EH) as well as subjects with normal blood pressure values (control) who have similar age and cardiovascular risk profile. Methods. Consecutively, 42 untreated ISH patients, 30 patients with EH and 29 normal subjects were included in the study. Parameters were presented as median (interquartile range). Results. There were no significant differences regarding age, gender and other cardiovascular risk factors among groups. As expected, systolic, diastolic blood pressure and pulse pressure values were significantly different among groups. Besides, PRA values were found to be significantly lower in patients with ISH (0.4 [0.2–1.1] ng/ml/h) compared with the EH (0.95 [0.5–2.6] ng/ml/h, p =0.024) and control (1.3 [0.7–2.1] ng/ml/h, p =0.001) groups. Although, PAL were similar among groups, PAL/PRA ratio was significantly higher in ISH group (134.1 [73–224]) compared with those with EH (42.2 [35–84], p <0.001) and the control group (53.3 [30–106], p =0.001). No significant difference was present with respect to PAL/PRA ratio between EH and control groups. Conclusions. Our findings suggested that in patients with ISH, despite lower PRA levels, PAL/PRA ratio is significantly higher compared with the patients with EH and subjects with normal blood pressure. Since higher PAL/PRA levels is an indicator of relative aldosterone excess, medications blocking RAAS activity including aldosterone antagonists may have useful cardiovascular consequences in addition to their antihypertensive effects in ISH.
Introduction
Worldwide, the prevalence of hypertension is increasing along with the aging of the general population (Citation1,Citation2). Although the prevalence of blood pressure (BP) elevation is particularly high in the elderly population, there are some unique features associated with raised BP in patients with advanced age. After the age of 50 or 60 years, diastolic BP tends to spontaneously decrease with increasing age while systolic BP increases, mainly as a result of the functional and structural changes in the arterial vasculature, namely arterial stiffness. (Citation3,Citation4) Isolated systolic hypertension (ISH), defined as a raised systolic BP with normal or low diastolic BP, affects around half of people aged >60 years (Citation4). There is now compelling evidence from cross- sectional, longitudinal and randomized controlled trials showing that ISH confers a substantial cardiovascular risk (Citation5–7). Renin–angiotensin–aldosterone system (RAAS) activity was widely studied in various forms of hypertension (Citation8–10). Although, recent studies have shown that RAAS components play a major role in the increased arterial stiffness, there are scarce data regarding plasma renin activity (PRA), plasma aldosterone (PAL) and PAL/PRA ratio, specifically in patients with ISH (Citation8–10). Simultaneous measurement of PAL, PRA and the PAL/PRA ratio are useful screening tool for primary aldosteronism (Citation11,Citation12). However, recent findings have suggested that in patients with resistant hypertension and normokalemia and not necessarily primary aldosteronism, higher values of aldosterone/renin ratio (thus relative aldosterone excess) is more frequent laboratory finding than expected (Citation13,Citation14).
Therefore, we aimed to determine PRA, PAL and PAL/renin ratio in patients (age >50 years) with newly diagnosed, untreated ISH and to compare these values with patients with essential hypertension (EH) as well as in subjects with normal BP values (controls) who have similar age and cardiovascular risk profile.
Subjects and methods
Study population
Between June 2008 and May 2009, among the patients aged >50 years evaluated in our outpatient clinic, 42 individuals who were newly diagnosed with ISH were included in the current study. During the same period, 30 subjects with a newly diagnosed EH and 29 individuals with normal BP as controls were recruited.
ISH was defined as a systolic BP >140 mmHg and diastolic BP <90 mmHg and EH as systolic BP >140 mmHg and diastolic BP >90 mmHg on two separate occasions (within 1 week). The BP was measured with the dominant arm after being seated for 5 min using a standard sphygmomanometer. Participants who have never used antihypertensive agents or discontinued antihypertensive medications for at least 3 weeks were included in the study. Baseline characteristics data were collected during hospital visits. History, physical examination and routine laboratory tests were used to exclude secondary hypertension
Patients aged <50 years and patients with any of the following conditions were excluded from our study: valvular heart disease, significant systemic disease, left ventricular dysfunction (echocardiographic left ventricular ejection fraction <50%), history of inflammatory disease and/or on anti-inflammatory medications, patients taking aldosterone antagonists and beta-adrenergic blockers at the time of study enrollment, anemia, hyperthyroidism and serum creatinine >1.4 mg/dl. This study received prior approval from the institutional ethics committee, and the procedures followed were in accordance with the institutional guidelines. All patients gave informed consent prior to being enrolled.
PAL and PRA measurement
The PAL and PRA were measured in a sitting position using standard techniques. The sampling was performed in the morning between 08:00 and 10:00 h after 12 h fasting. Briefly, PAL was measured using a commercial radioimmunoassay kit (Immunotech, Marseille, France). The PRA was measured by RIA of angiotensin I generated after incubation of the plasma sample in standardized conditions (Renin Maia, Adaltis, Italy). The lower limits of the serum aldosterone and PRA were 6 pg/ml and 0.1 ng/ml/h, respectively.
Statistical analysis
Continuous variables are presented as median (interquartile range) and categorical variables as percentage. The Kruskal–Wallis test was used with post hoc analysis to compare for parametric variables and chi-square test was used for comparisons of categorical data among groups. Spearman rank correlation analysis was used to assess the relationship between PAL/PRA ratio and BP variables. The SPSS 17.0 (Chicago, IL.) statistical software package was used for all computations. A two tailed p-value of <0.05 was considered statistically significant.
Results
Clinical characteristics
The baseline demographic and clinical characteristics of the study groups were well matched and are summarized in . There were no significant differences among groups regarding age. Also, cardiovascular risk profile was similar among groups. In addition to fasting blood glucose and serum lipid profile, there were no significant differences among groups in serum electrolytes (including sodium, potassium). Hemoglobin and thyroid stimulating hormone levels were also similar.
Table I. Demographic and laboratory characteristics of study groups.
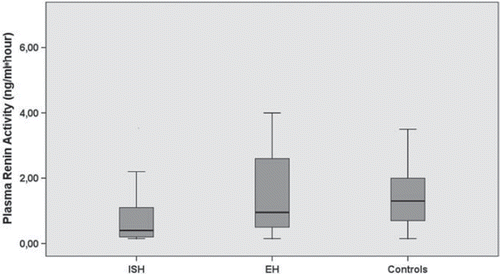
BP and laboratory measurements including PRA, PAL and PAL/PRA ratio are presented in . As expected, there were significant differences among groups in systolic as well as diastolic BP values. Systolic BP was significantly higher in both hypertensive groups compared with controls (p <0.001 for both comparisons). No significant difference was detected between ISH and EH group regarding systolic BP (p =0.3). Diastolic BP values were significantly higher in essential HT group compared with ISH and controls (p <0.001 for both comparisons). However, marginally significant difference was detected between ISH group and controls regarding diastolic BP (p =0.055). The highest pulse pressure value was seen in patients with ISH.
Table II. Blood pressure and plasma renin activity, aldosterone parameters.
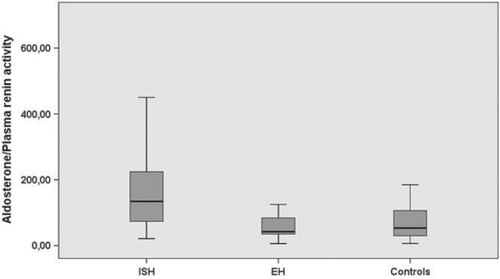
Although PRA values were similar between essential HT group and controls (p =0.2), it was significantly lower in patients with ISH compared with those with essential HT (p =0.024) and controls (p =0.001) (). Among groups no statistically significant difference was present regarding PAL (p =0.091). However, PAL/PRA ratio was significantly higher in patients with ISH compared with both essential HT group (p <0.001) and controls (p =0.001) (). PAL/PRA ratio was similar between essential HT group and controls. There were moderate but significant correlation between PAL/PRA ratio and systolic BP (r =0.23, p =0.02) as well as pulse pressure (r =0.34, p =0.001). However, no significant relationship was found between PAL/PRA ratio and diastolic BP (r = − 0.1, p =0.3).
Discussion
In the present study, we have found that PRA was significantly lower and PAL/PRA ratio was significantly higher in patients with newly diagnosed and untreated ISH compared with those with EH and subjects with normal BP. Therefore, our findings have suggested that ISH, a significant result of increased arterial stiffness, is associated with relative aldosterone excess.
By aging, ISH becomes the predominant form of increased BP. There are some unique features associated with the age-related increase in BP such as low PRA and increased arterial stiffness (Citation3,Citation10). Also, aldosterone might have a significant contribution to HT in the elderly (Citation15). Increased pulse pressure, which is a consequence of ISH and increased large artery stiffness, is a better predictor of cardiovascular events than systolic or diastolic pressure alone in people aged >50 years (Citation16). In our study, only patients with aged >50 years were included and they were normokalemic. Despite this, they had low PRA and relatively higher aldosterone levels. Simultaneous measurement of PAL, PRA and the aldosterone/renin ratio are being used as a useful screening test for primary aldosteronism (Citation11,Citation12). Since primary aldosteronism is characterized by increased aldosterone with consequent extracellular volume expansion and suppression of PRA, individuals with the disorder should have higher values of the ratio than those with EH. However, its sensitivity and specificity have not been determined in individuals with presumed EH or specifically ISH (Citation13,Citation14). Interestingly, even within normal ranges, higher levels of PAL have been demonstrated to be associated with higher risk of hypertension in the future in non-hypertensive population (Citation17). Recent studies have shown that numerous patients with resistant hypertension but not necessarily primary aldosteronism have lower PRA and normokalemia despite relative aldosterone excess. These groups might well respond mineralocorticoid receptor antagonists (Citation13,Citation14). The final step in RAAS, aldosterone has a major role in vascular remodeling. High aldosterone levels have been shown to lead to deleterious effects in arterial wall matrix by increasing collagen content and accumulation of fibronectin. Aldosterone also increases vascular muscle cell hypertrophy and inflammation of the vessel wall (Citation18–20). Besides, mineralocorticoid receptors have been demonstrated in the aorta (Citation21). In animal models, aldosterone has been shown to increase arterial stiffness and pulse pressure, which can be prevented with mineralocorticoid receptor antagonist, eplerenone (Citation19). Increased pulse wave velocity, a marker of increased large artery stiffness is clearly known to associate with higher risk of cardiovascular morbidity and mortality. Park et al. (Citation22) has shown that in hypertensive population, serum aldosterone is significantly associated with the central aortic pulse wave velocity. Accordingly, in our study, PAL/PRA ratio was found to be significantly correlated with the systolic BP as well as pulse pressure but not with diastolic BP. Increased pulse pressure is a consequence of increased large artery stiffness. Therefore, agents that block the RAAS might have more beneficial effects than other antihypertensive classes in patients with systolic HT. Indeed, in the LIFE study, which is conducted in elderly patients with ISH, a RAAS-blocking agent losartan had a relative risk reduction in cardiovascular outcomes compared with atenolol despite similar peripheral BP reduction seen in both agents. This was thought to be result of the greater reduction in central BP by losartan compared with atenolol (Citation23). Also, a selective aldosterone antagonist eplerenone has been shown to reduce systolic BP and pulse pressure as effective as amlodipine in patients with ISH aged >50 years. Besides, eplerenone has been found to be more effective in reducing microalbuminuria than amlodipine (Citation24). Black patients with EH share the same features as the ISH encountered in the elderly population. Low PRA and relative excess in aldosterone levels have been demonstrated in blacks with increased BP (Citation25). Interestingly, black hypertensives have significantly higher prevalence of left ventricular hypertrophy and concentric LV remodeling than do their white counterparts, which could be attributed to relative excess in aldosterone levels (Citation26).
Study limitations
In addition to the small sample size, there are some limitations in this study. First, aldosterone secretions show fluctuations during the day. However, we only obtained 12-h fasting blood samples to measure PRA and aldosterone. Multiple sampling may be more appropriate. Also, although ISH is a consequence of increased aortic stiffness, we neither directly evaluated the central aortic pressure invasively nor assessed the aortic pulse wave velocity. Incorporating such data may give much more valuable information in both hypertension groups as well as controls regarding the PAL/PRA ratio and arterial elasticity.
In conclusion, we found that untreated ISH and normokalemia is associated with high PAL/PRA ratio. Since higher PAL/PRA levels are an indicator of relative aldosterone excess, medications blocking RAAS activity including aldosterone antagonists may have useful cardiovascular consequences in addition to their antihypertensive effects in ISH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, . Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, .; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289: 2560–2572.
- Lakatta EG. Central arterial aging and the epidemic of systolic hypertension and atherosclerosis. J Am Soc Hypertens. 2007;1:302–340.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med. 2007;357:789–796.
- Ramsay LE, Williams B, Johnston GD, MacGregor GA, Poston L, Potter JF, . Guidelines for management of hypertension: Report of the third working party of the British Hypertension Society. J Hum Hypertens. 1999;13:569–592.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: Final results of the systolic hypertension in the elderly program. JAMA. 1991;265:3255–3265.
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, . Randomised doubleųblind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764.
- Mahmud A, Feely J. Arterial stiffness and the renin– angiotensin–aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5:102–108.
- Mohamed MA, Weir MR. Renin angiotensin system inhibition in the older person: A review. Clin Geriatr Med. 2009;25: 245–257.
- Kithas PA, Supiano MA. Practical recommendations for treatment of hypertension in older patients. Vasc Health Risk Manag. 2010;6:561–569.
- Hiramatsu K, Yamada T, Yukimura Y, Komiya I, Ichikawa K, Ishihara M, . A screening test to identify aldosterone producing adenoma by measuring plasma renin activity: Results in hypertensive patients. Arch Intern Med. 1981;141: 1589–1593.
- Moneva MH, Gomez-Sanchez CE. Establishing a diagnosis of primary aldosteronism. Curr Opin Endocrinol Diabetes. 2001;8:124–129.
- Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: A clue to efficient treatment. J Hypertens. 2004;22:2217–2226.
- Tomaschitz A, Maerz W, Pilz S, Ritz E, Scharnagl H, Renner W, . Aldosterone/renin ratio determines peripheral and central blood pressure values over a broad range. J Am Coll Cardiol. 2010;55:2171–2180.
- Sowers JR, Whaley-Connell A, Epstein M. Narrative review: The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783.
- O’Rourke MF, Frohlich ED. Pulse pressure: Is this a clinically useful risk factor? Hypertension. 1999;34:372–374.
- Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, . Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41.
- Duprez D, De Buyzere M, Rietzschel ER, Clement DL. Aldosterone and vascular damage. Curr Hypertens Rep. 2000;2:327–334.
- Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: Effects of eplerenone. Circulation. 2002;106:2848–2853.
- Nehme JA, Lacolley P, Labat C, Challande P, Robidel E, Perret C, . Spironolactone improves carotid artery fibrosis and distensibility in rat post-ischaemic heart failure. J Mol Cell Cardiol. 2005;39:511–519.
- Schmidt BM, Schmieder RE. Aldosterone-induced cardiac damage: Focus on blood pressure independent effects. Am J Hypertens. 2003;16:80–86.
- Park S, Kim JB, Shim CY, Ko YG, Choi D, Jang Y, . The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J Hypertens. 2007;25:1279–1283.
- Kjeldsen SE, Dahlöf B, Devereux RB, Julius S, Aurup P, Edelman J, .; LIFE (Losartan Intervention for Endpoint Reduction) Study Group. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: A Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–1498.
- White WB, Duprez D, St Hillaire R, Krause S, Roniker B, Kuse-Hamilton J, . Effects of the selective aldos- terone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41: 1021–1026.
- He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin–angiotensin system and blood pressure: Differences between blacks and whites. Am J Hypertens. 1999; 12:555–562.
- El-Gharbawy AH, Nadig VS, Kotchen JM, Grim CE, Sagar KB, Kaldunski M, . Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–850.