Abstract
It is well established that hypertension and obesity appear to be associated. The exact mechanism by which they are linked is unclear and remains a topic of a great deal of research. Current NICE guidelines recommend that patients with a BMI in excess of 35 kg/m2 should be considered for bariatric surgery if they have a concomitant obesity-associated condition, of which hypertension is one. The commonest bariatric procedure in the UK is the Roux-en-Y gastric bypass, which has been shown to result in long-standing remission of hypertension in up to 93% of patients. This paper summarizes the existing literature on the main theories as to how obesity leads to hypertension as well as the literature concerning the effects of gastric bypass surgery on hypertension.
Key Words::
Introduction
The adverse health consequences of obesity are many and varied – almost every system in the body can be affected (Citation1,Citation2). A systematic review in 2010 suggested hazard ratios of between 1.1 and 5.1 for the development of cardiometabolic morbidity [type 2 diabetes mellitus (DM), hypertension, ischaemic heart disease and cerebrovascular disease] in adulthood amongst overweight or obese children as compared with children of normal weight (Citation3). Attempts to quantify the exact reduction in life expectancy imposed by obesity have varied; however, the generally accepted figure of 7 years would imply that obesity has as great an impact as lifelong smoking (Citation3–5).
The relationship between hypertension and adiposity has been well established in numerous studies over the past few decades across a variety of ethnicities and weight ranges (Citation6–9). In 1987, Garrison et al. (Citation8) estimated that up to 78% of cases of hypertension in men and 64% of cases in women may be directly attributable to patients either being overweight or obese (Citation8,Citation10). The connection between the two has been further clarified by such studies as Jones et al. (Citation11), who showed that there was a linear relationship between blood pressure (BP) and body mass index (BMI) – for every BMI increase of 1 kg/m2 in normal weight individuals the diastolic BP rose by 0.89 mmHg, whereas in overweight individuals it increased by 1 mmHg. Subsequent studies have shown a similar correlation (Citation7). Having said all this, it is worth remembering that despite the increased prevalence of hypertension in the obese population, not all obese patients are hypertensive and not all hypertensive patients have a raised BMI (Citation6,Citation12). Although even modest BMI increases and decreases can result in corresponding changes in BP, in both the normal weight and overweight/ obese populations, there is still considerable inter-individual variability (Citation6,Citation12–14).
Pathophysiology of obesity-related hypertension
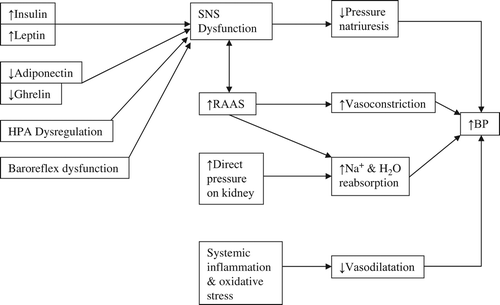
Exactly how obesity leads to hypertension is not fully understood; however, several overlapping theories have been proposed. Central to these theories is the concept that the adipose tissue of overweight or obese individuals is “dysfunctional” (Citation10,Citation15). This means that the tissue differs from normal adipose tissue by showing increased levels of adipocyte hypertrophy and macrophage infiltration and an altered secretory function of the adipocyte-released hormones (adipokines) (Citation10,Citation15). It is primarily this altered adipokine secretion component that underpins the hypotheses explaining the aetiology of obesity-associated hypertension.
Sympathetic nervous system dysfunction
The main proposed mechanism stems from the proven link between elevated body fat levels and overstimulation of the sympathetic nervous system (SNS) (Citation6,Citation12,Citation16–18). Activation of the SNS may induce hypertension by inducing peripheral vasoconstriction, impairing the urinary excretion of sodium and water and by stimulation of the renin–angiotensin–aldosterone system (RAAS) (Citation10). It should be borne in mind, however, that SNS activity may occur regionally or systemically and much of the literature on this topic is based on the erroneous assumption that systemic markers of SNS activity, such as those of skeletal muscle, are equal in effect to the SNS activity specific to those organs controlling BP, most notably the kidneys (Citation12,Citation19,Citation20). It has been suggested that obesity may not in fact result in systemic SNS hyperactivity but may instead result in a more selective SNS activation process – for example, skeletal muscle and renal SNS activity may be increased in obese patients, but cardiac SNS activity may be reduced due to baroreflex inhibition with the increased heart rate seen instead being a consequence of reduced parasympathetic activity (Citation12,Citation20–22). Those studies conducted since the development of more site-specific neurochemical and neurophysiological techniques have been pivotal in enhancing our understanding of the pathophysiology of obesity-related hypertension (Citation19).
As a result of these site-specific techniques, there is now good evidence clarifying the link between body fat levels and SNS activity (Citation19,Citation20). Several studies have shown that obese humans demonstrate up to twice the levels of post-ganglionic muscle SNS activity compared with non-obese subjects (Citation13,Citation17,Citation19,Citation20,Citation23–25). It has also been shown that ethnicity plays a role in the relationship between SNS activity, obesity and BP (Citation10,Citation26). An example of this finding would be Weyer et al. (Citation26), who found that muscle SNS activity positively related to body fat percentage in Caucasians in whom obesity and hypertension are both widely prevalent but not Pima Indians in whom levels of obesity are high but levels of hypertension are low. In addition, Alvarez et al. (Citation23) showed that SNS activity is more closely related to levels of intra-abdominal (visceral) than total fat mass or abdominal subcutaneous fat independently of total body fat (Citation6,Citation12,Citation19,Citation23,Citation27). This in turn would explain why such patients have also been found to have a greater association with hypertension and cardiovascular disease (Citation6,Citation12,Citation23,Citation27). The broad mechanisms linking obesity and SNS activity have been described in several review articles and are briefly outlined below.
Hyperleptinaemia
Leptin secretion by adipocytes occurs proportionately to fat mass and its levels are therefore raised in obese subjects (Citation12,Citation28–30). It acts primarily on receptors in the hypothalamus resulting in decreased appetite and increased peripheral thermogenesis (Citation6,Citation12,Citation16). Epidemiological evidence, studies of patients with congenital leptin deficiency and animal studies have also suggested a role for leptin in renal SNS activation and increased BP in the long-term if not acutely (Citation12,Citation16,Citation31–33). It appears to exert its central effects on the SNS by acting on receptors, which form parts of other CNS systems such as the melanocortin receptors in the anterior pituitary gland (Citation12,Citation34). It is thought that obese subjects may be selectively resistant to leptin's effects on weight control but not to its influence on renal SNS activity; however, the exact mechanism for this has yet to be proven (Citation16,Citation35).
Hypoadiponectinaemia
High molecular weight adiponectin has been shown to have a significant cardio-protective effect in terms of lowering BP and reducing the incidence of atheromatous plaque formation (Citation12,Citation36). In contrast to most adipocyte-secreted hormones, however, its levels in obesity are reduced (Citation36–38). Exactly how adiponectin reduces BP is not known but studies on rats have shown a dose-dependent reduction in renal SNS function when given either intravenously or intraventricularly (Citation12,Citation39). In addition, adiponectin has been shown to stimulate the actions of endothelial nitric oxide synthase thus inducing a reduction in vascular tone and smooth muscle proliferation (Citation10,Citation36,Citation40,Citation41). The elevated levels of free fatty acid and tumour necrosis factor-α seen in obesity are thought to impair nitric oxide synthase function and thus contribute to increased BP in a parallel fashion to the effects of hypoadiponectinaemia (Citation10,Citation42).
Hypoghrelinaemia
Ghrelin produced in the stomach and pancreas increases during fasting and appears to trigger the sensation of hunger. Rodent studies have suggested that it also counteracts the effects of leptin on melanocortin receptors thus inhibiting renal and systemic SNS activity (Citation43,Citation44). Ghrelin has also been shown to increase endothelial nitric oxide production thus resulting in reduced vascular tone (Citation45). Low ghrelin levels would therefore result in less central sympatho-inhibition and less systemic vasodilatation with a consequential increase in systemic BP.
Insulin resistance/hyperinsulinaemia
Although the evidence implicating insulin resistance in obesity-related hypertension is fairly weak, it has been postulated that the two may be indirectly linked by the effects of the former in terms of arterial intimal damage or chronic lipid metabolism dysfunction (Citation6). The main arguments against hyperinsulinaemia playing a role in hypertension in the acute or subacute settings are that studies of BP in animals in whom insulin has been infused intravenously or directly into the brain do not show a concomitant sustained rise in BP (Citation6,Citation12,Citation46,Citation47). Equally patients on therapeutic intravenous insulin drips or with proven insulinomas do not show a tendency towards hypertension (Citation48,Citation49).
Baroreflex dysfunction
Baroreflex function has been shown to be impaired in obesity, particularly visceral obesity, and there is evidence that this phenomenon is another action attributable to leptin (Citation21,Citation27,Citation50–54). However, since the baroreceptor reflex acts primarily to maintain acute BP stability, there remains some doubt as to the degree of influence this mechanism may exert on the BP of obese individuals in the long-term (Citation6,Citation12).
Hypothalamic–pituitary axis (HPA) dysregulation
It has been suggested that simultaneous activation of the SNS and HPA may play a role in the development of obesity-related hypertension (Citation6,Citation55). Evidence to support this hypothesis came in 2001 when Grassi et al. (Citation56) demonstrated that prolonged glucocorticoid administration resulted in SNS inhibition in obese but not normal weight subjects concluding that the HPA may affect SNS function in several ways (Citation6,Citation56). Since these early findings, it has been suggested that a variety of other metabolites, for example reactive oxygen species, may act on HPA pathways to induce sympatho-excitation with subsequent increases in BP (Citation57).
Renin–angiotensin–aldosterone system dysfunction
The second major hypothesis linking obesity to hypertension, related to the overstimulation of the SNS, is the concomitant increased activity in obesity of the RAAS, which brings about rises in BP via a variety of hormones primarily by directly augmenting renal sodium and water reabsorption and systemic vascular tone (Citation10,Citation58,Citation59). One of the major RAAS components, angiotensin II, has also been shown indirectly to increase BP acutely through central excitation of the SNS and baroreceptor reflexes (Citation6,Citation60–62). Paradoxically though, the effect on SNS function appears to reverse on chronic exposure (Citation63). Although the RAAS hormones are mainly secreted from organs other than adipose tissue, nearly all of them are elevated in obesity and reduce in concentration following bodyweight loss (BWL) (Citation10,Citation58,Citation64–69). It is clear that with the elevated levels of the RAAS hormones in obesity, a direct effect on BP could occur; however, the role of the RAAS on the SNS remains uncertain (Citation10).
Systemic inflammation and oxidative stress
The third major hypothesis linking hypertension to overweight and obesity centres on the well established association between increased adiposity and elevated levels of systemic inflammation and oxidative stress (Citation10,Citation70,Citation71). It is thought that the common link between the two is that several of the pro-inflammatory cytokines and acute phase reactants have been shown to impair the vasodilatory function of vascular mediators such as nitric oxide (Citation10,Citation70,Citation72,Citation73). In addition, the direct pressure effect of the adipose tissue on the kidneys is thought to elevate BP by encouraging sodium and water retention (Citation64,Citation74). Both pathways would provide plausible explanations as to why visceral obesity is particularly associated with hypertension as patients with predominantly elevated levels of intra-abdominal fat have been shown to have increased levels of systemic inflammatory markers, oxidative stress and intra-abdominal pressure as compared with those with predominantly subcutaneous fat (Citation71,Citation75–77).
Roux-en-Y gastric bypass (RYGBP) and hypertension resolution
In comparison with the number of papers investigating its effects on BMI and DM there is a relative dearth of data concerning the influence that RYGBP may have on BP. The largest series of RYGBP on hypertensive patients was reported in 2003 by Sugerman et al. (Citation78), who investigated 1025 patients of whom 521 were hypertensive (defined as systolic BP ≥ 150 mmHg, diastolic BP ≥ 90 mmHg and/or the use of antihypertensive medication). At 1–2 years post-RYGBP, hypertension had resolved in 69% of these patients [excess BWL (eBWL) 66 ± 18%, 91% follow-up rate] with this figure falling to 66% at 5–7 years (eBWL 59 ± 24%, 50% follow-up rate) and 51% at 10–12 years (eBWL 52 ± 25%, 37% follow-up rate) (Citation78). The risk factors for non- resolution of hypertension were increased age, lower eBWL and African-American ethnicity (p < 0.001, p < 0.001 and p < 0.02, respectively) (Citation78). The, perhaps predictable, finding that hypertension is more likely to resolve or improve if more weight is lost echoes those of smaller similar studies (Citation79,Citation80). Interestingly, it has been shown that in the majority of post-RYGBP patients not only does BP tend towards normal levels but also that the natural circadian rhythm of BP is generally restored – a phenomenon that is typically impaired in obesity (Citation81).
Hinojosa et al. (Citation82) reported a mean eBWL of 66% in a cohort of 95 patients and a 46% complete resolution rate at 12 months post-RYGBP with a further 19% of patients showing some improvement in their hypertension. Analogous to the findings of several studies looking at factors associated with DM resolution Hinojosa et al. (Citation82) found that the duration since diagnosis of hypertension was an independent risk factor for resolution with the complete resolution group having a mean duration of 53 months vs 95 months in the non-resolution group (p = 0.001).
The only other published factor associated with successful resolution of hypertension following RYGBP is that of DM status. Carbonell et al. (Citation83) reported on a cohort of 3193 patients of whom 655 (20%) also had DM. Although the paper does not state how many of the 3193 patients were hypertensive preoperatively, it does conclude that those subjects without concomitant DM were significantly more likely to experience resolution of their hypertension than those with both conditions (74.4% vs 63.5%, p < 0.0001) (Citation83).
Given that there are a variety of mechanisms by which obesity is thought to induce hypertension, it is perhaps not unreasonable to think that there may be a variety of mechanisms by which post-bariatric surgery BWL may induce its remission. It used to be thought that BWL itself was the key factor driving the return to normotension; however, a time-course analysis published in 2009 showed that improvements in BP occur well before any appreciable BWL (Citation84,Citation85). Ahmed et al. (Citation84) found that in their cohort of 100 patients the systolic and diastolic BPs reduced by 9 and 7 mmHg respectively at 1 week post-RYGBP. These measurements fell by a further 6 and 2 mmHg, respectively, with an overall 88% hypertension resolution rate over the remaining 12-month follow-up period, suggesting that BWL itself is not the key determinant of BP normalization following RYGBP (Citation84). It is likely that the early improvements in BP, if not the long-term improvements, are the result of a hormonal mechanism (Citation84). One possible explanation was proposed by Sledzinski et al. in 2010 (Citation86) who reported a 40% increased in serum nitric oxide levels 6 months post-vertical banded gastroplasty, although clearly the differences in the nature of this procedure and the RYGBP make it difficult to draw confident parallels. Improvements in endothelial vasomotor function and aortic elasticity following RYGBP have been described in the literature; however, the significance of these findings with regard to long-term BP control is not clear (Citation87,Citation88).
Conclusion
Whilst the link between obesity and hypertension is well established, there continues to be some controversy regarding the exact nature of the association. That BWL, whether surgical or non-surgical, effects a change towards normotension appears to be equally well accepted but again the mechanisms by which it does so remain elusive. In comparison with BWL and DM remission, few attempts appear to have been made to identify factors predictive of hypertension remission. Given the disease burden, this would seem to be a worthy area for future research.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–579.
- Uwaifo G, Arioglu E. Obesity. www.emedicine.com/med/topic1653.htm; 2006.
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int J Obes (Lond). 2011;35:891–898.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037.
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann Intern Med. 2003;138:24–32.
- Davy KP, Hall JE. Obesity and hypertension: Two epidemics or one?Am J Physiol Regul Integr Comp Physiol. 2004; 286:R803–R813.
- Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: Consistency of their association across developing and developed countries. Int J Obes Relat Metab Disord. 2002;26:48–57.
- Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: The Framingham Offspring Study. Prev Med. 1987;16:235–251.
- Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med. 2002;162:1867–1872.
- Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13:17–26.
- Jones DW, Kim JS, Andrew ME, Kim SJ, Hong YP. Body mass index and blood pressure in Korean men and women: The Korean National Blood Pressure Survey. J Hypertens. 1994;12:1433–1437.
- da Silva AA, do Carmo J, Dubinion J, Hall JE. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep. 2009;11:206–211.
- Masuo K, Mikami H, Ogihara T, Tuck ML. Weight gain-induced blood pressure elevation. Hypertension. 2000;35:1135–1140.
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension. 2003; 42:878–884.
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971.
- Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: Mechanisms and clinical implications. Hypertens Res. 2011;35:4–16.
- Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640.
- Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005; 90:5998–6005.
- Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33:116–124.
- Smith MM, Minson CT. Obesity and adipokines: Effects on sympathetic overactivity. J Physiol. 2012;590(Pt 8): 1787–1801.
- Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–447.
- Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429.
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002; 106:2533–2536.
- Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542.
- Masuo K, Mikami H, Itoh M, Ogihara T, Tuck ML. Sympathetic activity and body mass index contribute to blood pressure levels. Hypertens Res. 2000;23:303–310.
- Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension. 2000;36:531–537.
- Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–2369.
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000; 62:413–437.
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671.
- Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann Intern Med. 2010; 152:93–100.
- Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278.
- Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56:623–628.
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018.
- Slominski AT. Proopiomelanocortin signaling system is operating in mast cells. J Invest Dermatol. 2006;126: 1934–1936.
- Rahmouni K. Leptin-induced sympathetic nerve activation: Signaling mechanisms and cardiovascular consequences in obesity. Curr Hypertens Rev. 2011;6:104–209.
- Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234.
- Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129–134.
- Ohashi K, Kihara S, Ouchi N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116.
- Tanida M, Shen J, Horii Y, et al. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med (Maywood). 2007;232:390–397.
- Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14.
- Tan KC, Xu A, Chow WS, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769.
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000; 49:1231–1238.
- Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43: 977–982.
- Matsumura K, Tsuchihashi T, Fujii K, Abe I, Iida M. Central ghrelin modulates sympathetic activity in conscious rabbits. Hypertension. 2002;40:694–699.
- Xu X, Jhun BS, Ha CH, Jin ZG. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192.
- Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: Role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S.
- Liu J, da Silva AA, Tallam LS, Hall JE. Chronic central nervous system hyperinsulinemia and regulation of arterial pressure and food intake. J Hypertens. 2006;24: 1391–1395.
- Sawicki PT, Baba T, Berger M, Starke A. Normal blood pressure in patients with insulinoma despite hyperinsulinemia and insulin resistance. J Am Soc Nephrol. 1992;3:S64–S68.
- Scherrer U, Owlya R, Trueb L. Sympathetic-nerve activity before and after resection of an insulinoma. N Engl J Med. 1996;335:1240–1242.
- Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension. 2009;54:1001–1008.
- Del Colle S, Milan A, Caserta M, et al. Baroreflex sensitivity is impaired in essential hypertensives with central obesity. J Hum Hypertens. 2007;21:473–478.
- Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity- induced hypertension. Hypertension. 2007;49:1307–1314.
- Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338.
- McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol. 2012.
- Bjorntorp P, Holm G, Rosmond R, Folkow B. Hypertension and the metabolic syndrome: Closely related central origin?Blood Press. 2000;9:71–82.
- Grassi G, Seravalle G, Dell’Oro R, et al. Participation of the hypothalamus-hypophysis axis in the sympathetic activation of human obesity. Hypertension. 2001;38:1316–1320.
- Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887.
- Engeli S, Bohnke J, Gorzelniak K, et al. Weight loss and the renin–angiotensin–aldosterone system. Hypertension. 2005; 45:356–362.
- Kotchen TA. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23:1170–1178.
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–E778.
- Matsukawa T, Gotoh E, Minamisawa K, et al. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol. 1991;261:R690–R696.
- Miyajima E, Shigemasa T, Yamada Y, Tochikubo O, Ishii M. Angiotensin II blunts, while an angiotensin-converting enzyme inhibitor augments, reflex sympathetic inhibition in humans. Clin Exp Pharmacol Physiol. 1999;26:797–802.
- Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension. 2010;55: 644–651.
- Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1004; discussion 1004–1005.
- Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7: 355–362.
- Saiki A, Ohira M, Endo K, et al. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708–713.
- Umemura S, Nyui N, Tamura K, et al. Plasma angiotensinogen concentrations in obese patients. Am J Hypertens. 1997; 10:629–633.
- Grossman E, Eshkol A, Rosenthal T. Diet and weight loss: Their effect on norepinephrine renin and aldosterone levels. Int J Obes. 1985;9:107–114.
- Sowers JR, Nyby M, Stern N, et al. Blood pressure and hormone changes associated with weight reduction in the obese. Hypertension. 1982;4:686–691.
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761.
- Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation. 2007;116:1234–1241.
- Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–986.
- Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34: 665–673.
- Bloomfield GL, Sugerman HJ, Blocher CR, Gehr TW, Sica DA. Chronically increased intra-abdominal pressure produces systemic hypertension in dogs. Int J Obes Relat Metab Disord. 2000;24:819–824.
- Gletsu-Miller N, Hansen JM, Jones DP, et al. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity (Silver Spring). 2009;17:439–446.
- Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225–1232.
- Varela JE, Hinojosa M, Nguyen N. Correlations between intra-abdominal pressure and obesity-related co-morbidities. Surg Obes Relat Dis. 2009;5:524–528.
- Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237:751–756; discussion 757–758.
- Carson JL, Ruddy ME, Duff AE, Holmes NJ, Cody RP, Brolin RE. The effect of gastric bypass surgery on hypertension in morbidly obese patients. Arch Intern Med. 1994; 154:193–200.
- Jamal M, Samuel I, Heitshusen DI, Maher J. Resolution of hypertension follows surgical weight loss in patients undergoing gastric bypass surgery: A five year analysis of data. 27th Annual Meeting of the American Society for Metabolic and Bariatric Surgery. Las Vegas, NV: Surgery for Obesity and Related Diseases; 2010. p. S76.
- Czupryniak L, Strzelczyk J, Pawlowski M, Loba J. Circadian blood pressure variation in morbidly obese hypertensive patients undergoing gastric bypass surgery. Am J Hypertens. 2005;18:446–451.
- Hinojosa MW, Varela JE, Smith BR, Che F, Nguyen NT. Resolution of systemic hypertension after laparoscopic gastric bypass. J Gastrointest Surg. 2009;13:793–797.
- Carbonell AM, Wolfe LG, Meador JG, Sugerman HJ, Kellum JM, Maher JW. Does diabetes affect weight loss after gastric bypass?Surg Obes Relat Dis. 2008;4:441–444.
- Ahmed AR, Rickards G, Coniglio D, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg. 2009;19:845–849.
- Bueter M, Ahmed A, Ashrafian H, le Roux CW. Bariatric surgery and hypertension. Surg Obes Relat Dis. 2009;5: 615–620.
- Sledzinski T, Sledzinski M, Smolenski RT, Swierczynski J. Increased serum nitric oxide concentration after bariatric surgery – A potential mechanism for cardiovascular benefit. Obes Surg. 2010;20:204–210.
- Gokce N, Vita JA, McDonnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268.
- Ikonomidis I, Mazarakis A, Papadopoulos C, et al. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: A 3-year follow-up study. J Hypertens. 2007;25:439–447.