Abstract
Myeloperoxidase (MPO) is a member of the mammalian peroxidase superfamily and plays specific roles in host defense. This study aimed to explore the association between one polymorphism of MPO and hypertension risk. Study subjects were recruited from Taipei City Hospital, Zhongxiao Branch, Taipei Medical University Hospital and Taipei Municipal WanFang Hospital. Participants completed questionnaires and provided blood samples. In this study we considered hypertension to be present among subjects that had blood pressures above 140/90 mmHg, or who had previously received treatment for hypertension. The polymorphism of MPO investigated in this study was constructed by performing a restriction fragment length polymorphism following polymerase chain reaction. This study found the odds ratio and 95% confidence interval for hypertension among subjects with the MPO -463 GA/AA genotype to be 1.97 (1.23–3.16) when compared with those with the GG genotype after multivariate adjustment. Participants with a body mass index (BMI) ≥ 24 kg/m2 and with MPO -463 GA/AA genotype had a 4.60-fold increased risk of hypertension compared with those with a BMI < 24 kg/m2 and with the GG genotype. This is the first study to conclude that the MPO -463 GA/AA genotype was associated with hypertension. In addition, we also detected that subjects with the MPO -463 GA/AA genotype that had higher BMIs and positive diabetes status tended to have higher risks of hypertension than subjects with the MPO -463 GA/AA genotype that had normal BMIs and were not diabetic.
Key Words::
Introduction
Myeloperoxidase (MPO) is a hemeprotein excreted by neutrophils and monocytes when they are activated during inflammation. Furthermore, these processes generate superoxide, which can be converted to hydrogen peroxide by superoxide dismutase. Hydrogen peroxide is utilized by MPO to create an array of potent oxides including hypochlorous acid, hydroxyl radical, nitrogen dioxide and peroxynitrite (Citation1). These oxidants, termed oxidative biomarkers (Citation2), can interact with both low- and high-density lipoproteins to induce oxidation (Citation1) and propagate atherosclerosis (Citation3,Citation4). A functional promoter polymorphism has been identified in the promoter region of the MPO gene, consisting of a G to A substitution (Citation5). The -463G→A polymorphism is situated within an Alu-encoded hormone response element and creates a SP1 site in the G allele promoter, as well as an estrogen receptor binding site in the A promoter. Two alleles of the MPO gene exist that differ at one position within this element, resulting in one allele with and one allele without a strong SP1 binding site. The element with the SP1 site increases the activation of transcription by 25-fold in transient transfection assays, while the alternative allele confers several fold less transcriptional activity (Citation5). MPO-463G/A polymorphism can serve as a useful marker of atherosclerosis and cardiovascular events in dialysis patients (Citation6). The levels of MPO in neutrophils and serum were associated with the presence of angiographically demonstrated coronary atherosclerosis (Citation7). In French Canadian patients, the MPO-463 GG genotype was associated with an increased incidence of coronary artery disease (Citation8). MPO-463 G → A SNP, which supposedly results in lower MPO activity, is associated with a lower prevalence of cardiovascular disease in end-stage renal disease patients (Citation9), and the presence of the A allele is associated with a lower frequency of cardiovascular disease. However, the association between the MPO-463 G → A polymorphism and hypertension has not yet been determined. We hypothesized that the MPO-463 G/A polymorphism may have an effect on the risk of hypertension.
Methods
Study subjects
We conducted a hospital-based case–control study. All our study subjects were recruited from September 2005 to December 2006 from outpatient visits at the Taipei City Hospital, Zhongxiao Branch. In this study, we did not include patients with cancer. Age- and gender-matched controls who received senior citizen health examinations at the Taipei Medical University Hospital, and those who received adult health examinations at the Taipei Municipal WanFang Hospital. These two hospitals provide very similar services, have similar client bases and are both located in Taipei within approximately 4 km from each other. This study was approved by the ethics committee of Taipei City Hospital, Zhongxiao Branch. The study was consistent with the World Medical Association Declaration of Helsinki.
Questionnaire interview and biological specimen collection
Well-trained personnel described the purpose of this study for study subjects, received informed consent from the subjects, and then carried out standardized personal interviews based on a structured questionnaire and collected specimens. Information obtained included socio-demographic characteristics, lifestyle (cigarette smoking and consumption habits of alcohol, tea, coffee and other beverages), occupational and environmental exposure to possible carcinogens, and personal and familial disease history, including hypertension and diabetes. Detailed information was obtained on cigarette smoking including the age at which the subject began smoking cigarettes, as well as the average number of cigarettes smoked per day, and, if applicable, the age cigarette smoking cessation. Frequent alcohol, tea and coffee drinkers in this study were defined as those who consumed the respective beverages two or more days per week for at least 6 months. Those who consumed less than this level were classified as occasional drinkers. A 10-ml blood sample was collected from each subject upon recruitment, by use of EDTA-treated vacuum syringes and disposable needles. Plasma samples were centrifuged at 3000 rpm for 15 min at room temperature, separated into aliquots and stored at − 80°C until used. Buffy coat was prepared for DNA extraction, and the plasma was tested for blood sugar and lipid profile.
Blood pressure measurement, diagnosis of hypertension and definition of diseases
In this study, we followed the standard protocol recommended by the World Health Organization for measuring blood pressure. After subjects had rested for at least 20 min, both systolic and diastolic blood pressures (SBP and DBP) were measured with the subjects in a seated posture three times with a mercury sphygmomanometer. SBP and DBP were defined at the first and fifth Korotkoff sounds, respectively. The average of the three measurements was used for analysis. As a SBP of more than 140 mmHg is a much more important cardiovascular disease risk factor than DBP among patients older than 50 years of age (Citation10), in this study we defined subjects as hypertensive if their blood pressures was above 140/90 mmHg or if they had a history of regular treatment with antihypertensive drugs (Citation10). All the subjects in this study were primary hypertensive patients. Control subjects were defined as those having blood pressures below 140/90 mmHg (Citation10). Subjects were classified as diabetic if they were treated for insulin or non-insulin-dependent diabetes, or if they had a fasting glucose of > 126 mg/dl. Positive cardiovascular history was defined as having a history of myocardial infarction, angioplasty, coronary artery by-pass surgery, lower-limb arterial disease, stroke or transient ischemic attacks.
Anthropometric measurements
Standing height and body weight were measured with subjects’ barefoot and wearing light clothing. A rigid vertical height measurement tool and a standard medical scale were used. Height was measured to the nearest 0.5 cm and weight to the nearest 100 g. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. According to the definition of the Department of Health, Executive Yuan, Taiwan, a subject with a BMI between 24.0 and 27.0 is classified as overweight, and 27.0 or higher as obese (Citation11).
Genotyping
We detected the MPO-463G→A polymorphism by performing a restriction fragment length polymorphism (RFLP) after polymerase chain reaction (PCR), as described by London et al. (Citation12). A 350-bp DNA fragment was amplified using forward primer MPOF (5´-CGG TAT AGG CAC ACA ATG GTG AG) and reverse primer MPOR (5´-GCA ATG GTT CAA GCG ATT CTT C). PCR was performed and 10 μl of the PCR product was digested with the restriction enzyme AciI. After electrophoresis, the digested products resulted in banding patterns indicative of the genotypes. These included 169-, 120- and 61-bp fragments for the homozygous wild-type (-463GG), 289-, 169-, 120- and 61-bp fragments for the heterozygous type (-463AG), and 289- and 61-bp fragments for the homozygous mutant (-463AA).
Statistical analysis
All the statistical analyses performed in this study were carried out with the SAS program (version 9.2). All results for continuous variables were expressed as mean± standard error (SE). The t-test was used to explore differences between two groups for continuous variables. Comparisons between exposures, measured as a categorical variable, and Hardy–Weinberg equilibrium for genotype distributions of controls were assessed by χ2 analysis. ANOVA and Duncan's multiple comparisons test were used to compare continuous variables between varied genotype strata. The relationship between the genotypes and hypertension status was investigated through the construction of logistic regression models, which we used to generate estimated odds ratios (OR) and 95% confidence intervals (CI). Probability values < 0.05 were considered statistically significant. The significant variables in the univariate analysis were included in the multivariate logistic regression models. The joint effects of diabetes and MPO G-463A and the joint effects of BMI and MPO G-463A on the risk of hypertension were evaluated on both multiplicative and additive scales. The binary interaction terms were calculated by multiplying the indicators for the two explored risk factors, and were added to the main effect models. Their significance was then tested by the likelihood ratio statistic based on a multiplicative model. The OR values and their variance–covariance matrix were then used to calculate values for the synergy index and 95% CIs (Citation13).
Results
The socio-demographic characteristics of the hypertension patients and normotensive participants analyzed in this study are shown in . A total of 508 subjects, including 254 hypertensive patients and 254 normotensive participants, were recruited in this study and 59.6% were male (). BMI was significantly associated with hypertension risk in a dose–response relationship (trend test, p < 0.001). Participants with an educational attainment of college or above had lower ORs for hypertension than those with only a junior high school level education or below. Therefore, educational attainment seemed to provide a protective effect against hypertension. Participants with diabetes had significantly higher ORs than non-diabetics (5.70, 95% CI 2.81–11.56). Current alcohol drinkers, and occasional tea and coffee drinkers had significantly lower hypertension risks than non-drinkers. The distributions of the MPO-463G → A polymorphism of controls were fitted to the Hardy–Weinberg equilibrium. The distribution of the MPO gene polymorphism was similar between hypertensive and normotensive persons.
Table I. Socio-demographic characteristics of hypertension patients and normotension subjects.
The participants in this study with the MPO AA genotype were on average younger than those with either the GG or GA genotype. BMI, SBP, DBP, lipid profile including triglycerides, cholesterol, low-density and high-density lipoproteins (HDL), and fasting blood glucose were similar among varied genotypes (data not shown). However, higher triglycerides, cholesterol and fasting blood glucose levels were positively associated with hypertension risk, whereas HDLs were negatively associated with hypertension risk (data not shown).
We tried to control for significant risk factors of hypertension in our analysis of the association between the MPO G-463A polymorphism and hypertension risk. It was found that increased serum HDL levels were associated with a lower risk of hypertension and that BMI and diabetes were significantly related with the presence of hypertension. Participants with the MPO GA/AA genotype had significantly higher ORs than those with the GG genotype in the multivariate model (). The results were similar when further adjusted for alcohol, tea and coffee drinking.
Table II. Multivariate analysis between MPO G-463A polymorphism and hypertension risk.
The associations between MPO genotype and hypertension stratified by cigarette smoking and diabetes status are summarized in . Non-smokers who carried the MPO GA/AA genotype had a significantly higher risk (OR 2.43, 95% CI 1.34–4.42) than those who carried the MPO GG group after multivariate adjustment. However, this phenomenon was not evident among cigarette smokers. On the other hand, non-diabetics with the MPO GA/AA genotype had a significantly higher adjusted risk (OR 1.98, 95% CI 1.21–3.24) than the MPO GG group, while there was no significantly increased risk detected among diabetic patients that carried the MPO GA/AA genotype.
Table III. MPO G-463A polymorphism and hypertension risk stratified by cigarette smoking status or diabetes status.
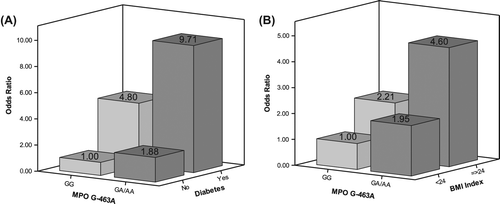
Since BMI, diabetes and the MPO G-463A genotype were significantly associated with hypertension in the multivariate analysis, further analyses were carried out to assess the joint effects of the MPO G-463A genotype and these two risk factors (BMI and diabetes) independently. We also performed a trend test for a dose–response relationship and found those subjects with MPO GA/AA genotype and either diabetes or a BMI ≥ 24 had an even elevated risk of hypertension than subjects with MPO GA/AA but without diabetes or a BMI < 24 ().
Discussion
In this study, diabetics and those subjects with lower levels of educational attainment had higher risk of hypertension than subjects who were non-diabetic and had higher levels of educational attainment. Participants that drank tea or coffee exhibited a decreased risk of hypertension. The participants that had high BMIs were at significantly increased risk of hypertension in a dose–response relationship. As previously reported, obesity is a major risk factor of hypertension (Citation14). In the current study, we provided more evidence that obesity (BMI ≥ 27 kg/m2) is a major risk factor of hypertension. Diabetes is another commonly cited risk factor associated with hypertension (Citation15). Increased fasting blood glucose levels and insulin resistance are both correlated with the occurrence of hypertension (Citation16). In this study, levels of fasting blood glucose were associated with hypertension in a significant dose–response relationship whether analyzed by stratification of clinic values (< 100, 100–126, > 126 mg/dl) or by the tertile of the control group (data not shown).
Previous studies on the association between cigarette smoking habits and blood pressure have been conflicting and controversial. For instance, in one report it was reported that there was no correlation between cigarette smoking and blood pressure (Citation17), in another that the blood pressure of smokers was lower than that of non-smokers (Citation18), and in another that cigarette smoking raised blood pressure and resulted in an accelerated heart rate (Citation19). In this study, participants that smoked more than 0.65 packs a day had a 1.5 times increased risk of hypertension than non-smokers. The duration of cigarette smoking was not related with hypertension in a dose–response manner (data not shown). Therefore, the chronic effect of cigarette smoking on hypertension may not be apparent in this study.
Hypertension increases vascular production of reactive oxygen species (ROS) in all layers of blood vessel walls. The increased vascular superoxide free radical production is in large part responsible for reducing endothelium-dependent vasodilation (Citation20). MPO is a member of the mammalian peroxidase superfamily and plays specific and complementary roles in host defense (Citation21). MPO generates the reactive chlorinating species hypochlorous acid (HOCl) (Citation22), which possesses potent microbicidal activity (Citation23). MPO induced reactive oxidants can initiate lipid peroxidation (Citation24) and modify target proteins through mechanisms including halogenation and nitration (Citation3,Citation4). One study reported that MPO might be involved in the regulation of inducible nitric oxide synthase (iNOS) expression, and found the expression of iNOS to be significantly higher in patients with the MPO G allele (61.54%, 48/78) than in patients with MPO A allele (38.46%, 30/78) (Citation25). It was suggested that the expression levels of iNOS and MPO were correlated with hepatopulmonary syndrome (HPS)-induced hypoxemia, and the MPO-463 G/A mutation might be a protective factor that prevents the development of HPS (Citation25). The association between MPO level and MPO polymorphism is controversial. The GA genotype is associated with 1.6–2.5-fold higher MPO mRNA levels than the GG genotype in primary human peripheral blood mononuclear cells (Citation26). In macrophages, the GG genotype has been shown to be associated with 4.6–7-fold higher MPO levels than the GA genotype (Citation26). One recent study showed that MPO levels were increased in coronary artery disease but the MPO-463 G/A polymorphism had no effect on MPO levels (Citation27). Thus, the MPO A allele can be higher or lower expressing than the MPO G allele, depending on the cell type. In this study, the MPO GA/AA genotype was significantly associated with hypertension risk after adjusting for multiple risk factors. This may suggest that the increased risk of hypertension among participants with the MPO GA/AA genotype may be mediated through interactions with NO and their effect on the inhibition of vasodilatation. Further investigation will be needed to explore the mechanism underpinning the increased risk of hypertension detected in this study.
The common MPO G-463A polymorphism has been associated with Alzheimer's disease (Citation28), lung cancer (Citation29) and brain damage (Citation30). The MPO -463A allele significantly contributes to a protective effect in smokers against lung cancer (Citation31) and individuals with the MPO GA/AA genotype have been shown to have a lower risk of experiencing a cardiovascular event (Citation32). A previous study found that the OR for coronary artery disease (CAD) of participants with the MPO AA genotype was 0.17 that of subjects with the MPO GG/AG genotype, suggesting that the A allele may have a significantly protective effect against CAD (Citation33). In contrast, another study showed no significant relationship between the MPO G-463A genetic polymorphism and brain infarction risk, but the MPO A allele was associated with the extent of brain damage (Citation30). Prior to the current study, however, there has been no report regarding the risk of hypertension among subjects with the MPO G-463A genetic polymorphism.
Analysis within the current study was performed by stratification of cigarette smoking status. Non-smoking participants with the MPO-463 GA/AA genotype carried a 2.43-fold higher risk than the GG genotype, but this increased risk was not significant in smokers following multivariate adjustment. We believe this finding suggests that cigarette smoking status may have had a more powerful effect on blood pressure than the MPO G-463A genotype. The potential increased hypertension risk in subjects with the variant A allele should be further studied.
The MPO G-463A genotype was also analyzed by stratifying on diabetes status. We found that in non-diabetic participants the OR of hypertension with the MPO-463 GA/AA genotype was significantly higher than those with the MPO-463 GG genotype; however, this association was higher but statistically insignificant in those with diabetes. In contrast, a study has reported that the presence of type 2 diabetes mellitus and the MPO-463 GG genotype had significant interactions on intima-media thickness (Citation34). However, further studies are needed to explore the mechanisms associated with the joint effect of diabetes and the MPO G-463A genotype on hypertension risk.
In this study, participants with the MPO-463 GA/AA genotype were shown to be associated with hypertension after controlling for potential risk factors for hypertension, such as BMI ≥ 24 kg/m2, lower level of educational attainment, diabetes and high fasting blood sugar. The reasons behind these increased risks need further examination, but may be due to increased ROS caused by obesity (Citation35) or high blood glucose (Citation36).
While the present study succeeded in detecting a novel association between hypertension and subjects carrying the MPO-463 GA/AA genotype, our results need to be interpreted with regard to several limitations. First, there is a possibility that this study suffered from selection bias as the cases and controls were recruited from two different hospitals; however, we feel that the bias should be minimal as these hospitals were both located in Taipei. Furthermore, the majority of cases and controls lived in Taipei and were similar in demographic characteristics. Second, because of the small sample size and lack of a replication population, these results should be interpreted with caution and warrant the confirmation of future studies.
In summary, to the best of our knowledge, this is the first study to conclude that the MPO-463 GA/AA genotype was associated with an increased risk of hypertension following multivariate adjustment. In addition, BMI ≥ 24 kg/m2 or diabetes tended to affect the hypertension risk induced by the MPO -463 GA/AA genotype.
Conflict of interest
We disclosed all financial and interpersonal relationships that could be viewed as presenting a potential conflict of interest.
The study was supported by grant from The study was supported by grants from the National Science Council of the ROC (NSC92-3112-B-038-001, NSC92-2321-B-038-004, NSC93-2321-B-038-012, NSC93-3112-B-038-001, NSC94-2314-B-038-023, NSC-95-2314-B-038-007, NSC-96-2314-B038-003, NSC 97-2314-B-038-015-MY3 (1-3), NSC 97-2314-B-038-015-MY3 (2-3) and NSC 97-2314-B-038-015-MY3 (3-3)).
References
- Tsimikas S. In vivo markers of oxidative stress and therapeutic interventions. Am J Cardiol. 2008;101:34D–42D.
- Strobel NA, Fassett RG, Marsh SA, Coombes JS. Oxidative stress biomarkers as predictors of cardiovascular disease. Int. J Cardiol. 2011;147:191–201.
- Heinecke JW. Oxidative stress: New approaches to diagnosis and prognosis in atherosclerosis. Am J Cardiol. 2003;91:12A–16A.
- Podrez EA, bu-Soud HM, Hazen SL. Myeloperoxidase- generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725.
- Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, Reynolds WF. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone–retinoic acid response element. J Biol Chem. 1996;271:14412–14420.
- Doi K, Noiri E, Maeda R, Nakao A, Kobayashi S, Tokunaga K, et al. Functional polymorphism of the myeloperoxidase gene in hypertensive nephrosclerosis dialysis patients. Hypertens Res. 2007;30:1193–1198.
- Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286: 2136–2142.
- Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J. 2001;142:336–339.
- Pecoits-Filho R, Stenvinkel P, Marchlewska A, Heimburger O, Barany P, Hoff CM, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003;S172–S176.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572.
- Department of Health, Taiwan Government. 2010; http://www.doh.gov.tw/CHT2006/other/ShowCopy.aspx?doc_no = 32&class_no = 25
- London SJ, Lehman TA, Taylor JA. Myeloperoxidase genetic polymorphism and lung cancer risk. Cancer Res. 1997; 57:5001–5003.
- Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology 1992;3:452–456.
- Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007;20:370–377.
- Bog-Hansen E, Lindblad U, Ranstam J, Melander A, Rastam L. Impaired glucose metabolism and obesity in Swedish patients with borderline isolated systolic hypertension: Skaraborg Hypertension and Diabetes Project. Diabetes Obes Metab. 2001;3:25–31.
- Fujita T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol Dial Transplant. 2007; 22:3102–3107.
- Simons LA, Simons J, Jones AS. The interactions of body weight, age, cigarette smoking and hormone usage with blood pressure and plasma lipids in an Australian community. Aust NZ J Med 1984;14:215–221.
- Okubo Y, Suwazono Y, Kobayashi E, Nogawa K. An association between smoking habits and blood pressure in normotensive Japanese men: A 5-year follow-up study. Drug Alcohol Depend. 2004;73:167–174.
- Cagirci G, Cay S, Karakurt O, Eryasar N, Kaya V, Canga A, et al. Influence of heavy cigarette smoking on heart rate variability and heart rate turbulence parameters. Ann Noninvasive Electrocardiol. 2009;14:327–332.
- Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am. 2009;93:621–635.
- Nauseef WM. Insights into myeloperoxidase biosynthesis from its inherited deficiency. J Mol Med 1998;76:661–668.
- Weiss SJ, Test ST, Eckmann CM, Roos D, Regiani S. Brominating oxidants generated by human eosinophils. Science 1986;234:200–203.
- Klebanoff SJ. Myeloperoxidase: Contribution to the microbicidal activity of intact leukocytes. Science 1970;169: 1095–1097.
- Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, et al. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J Biol Chem. 2009;284:3158–3169.
- Wang Y, Wang W, Zhang Y, Zhao X, Yang D. Clinical significance of a myeloperoxidase gene polymorphism and inducible nitric oxide synthase expression in cirrhotic patients with hepatopulmonary syndrome. J Huazhong Univ Sci Technol Med Sci. 2010;30:437–442.
- Kumar AP, Piedrafita FJ, Reynolds WF. Peroxisome proliferator-activated receptor gamma ligands regulate myeloperoxidase expression in macrophages by an estrogen- dependent mechanism involving the -463GA promoter polymorphism. J Biol Chem. 2004;279:8300–8315.
- Ergen A, Isbir S, Timirci O, Tekeli A, Isbir T. Effects of myeloperoxidase -463 G/A gene polymorphism and plasma levels on coronary artery disease. Mol Biol Rep. 2011;38:887–891.
- Pope SK, Kritchevsky SB, Ambrosone C, Yaffe K, Tylavsky F, Simonsick EM, et al. Myeloperoxidase polymorphism and cognitive decline in older adults in the Health, Aging, and Body Composition Study. Am J Epidemiol. 2006;163: 1084–1090.
- Taioli E, Benhamou S, Bouchardy C, Cascorbi I, Cajas-Salazar N, Dally H, et al. Myeloperoxidase G-463A polymorphism and lung cancer: A HuGE genetic susceptibility to environmental carcinogens pooled analysis. Genet Med. 2007;9:67–73.
- Hoy A, Leininger-Muller B, Poirier O, Siest G, Gautier M, Elbaz A, et al. Myeloperoxidase polymorphisms in brain infarction. Association with infarct size and functional outcome. Atherosclerosis. 2003;167:223–230.
- Arslan S, Pinarbasi H, Silig Y. Myeloperoxidase G-463A polymorphism and risk of lung and prostate cancer in a Turkish population. Mol Med Rep. 2011;4:87–92.
- Asselbergs FW, Reynolds WF, Cohen-Tervaert JW, Jessurun GA, Tio RA. Myeloperoxidase polymorphism related to cardiovascular events in coronary artery disease. Am J Med. 2004;116:429–430.
- Zhong C, Quanzhong Y, Genshan M, Hua Z, Ruolong Z, Jiahong W, et al. Myeloperoxidase gene-463G – A polymorphism and premature coronary artery disease. Genet Mol Biol. 2009;32:260–263.
- Makela R, Loimaala A, Nenonen A, Mercuri M, Vuori I, Huhtala H, et al. The association of myeloperoxidase promoter polymorphism with carotid atherosclerosis is abolished in patients with type 2 diabetes. Clin Biochem. 2008;41: 532–537.
- Colombani AL, Carneiro L, Benani A, Galinier A, Jaillard T, Duparc T, et al. Enhanced hypothalamic glucose sensing in obesity: Alteration of redox signaling. Diabetes. 2009; 58:2189–2197.
- Mellor K, Ritchie RH, Meredith G, Woodman OL, Morris MJ, Delbridge LM. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition. 2010;26:842–848.