Abstract
Background. Abdominal obesity, characterized by ectopic fat deposition in skeletal muscle and liver tissue, has been associated with insulin resistance and increased risk for type 2 diabetes mellitus. The aim of this study was to evaluate whether treatment with the angiotensin II type 1 (AT-1) receptor blocker telmisartan can reduce intramyocellular lipid (IMCL) and hepatic fat storage, thereby improving insulin sensitivity among individuals with abdominal obesity. Methods. Ninety-five adults with abdominal obesity (body mass index ≥ 30 kg/m2 and waist circumference > 102 cm in men and > 88 cm in women) were randomized to double-blind treatment with telmisartan or placebo for 24 weeks. Following 4 weeks of 80 mg telmisartan per day, the dose was increased to 160 mg telmisartan for the duration of the study. Soleus muscle IMCL and liver fat content were assessed by 1H-magnetic resonance imaging (1H-MRI) spectroscopy. Secondary outcomes included changes in body composition, plasma lipids, glucose profiles, insulin sensitivity, beta-cell function and total adiponectin levels. Results. There was no significant effect of telmisartan in abdominally obese individuals consuming either a low or high glycemic diet, on IMCL content (5.73 ± 1.11 vs 6.11 ± 1.11; p = 0.13) or liver fat (0.08 ± 0.05 vs 0.09 ± 0.05; p = 0.60). Body composition, lipid and glucose profiles, insulin sensitivity and adiponectin were likewise unaffected. Beta-cell function, as determined by the insulinogenic index (IGI), improved significantly (19.3 ± 13.7 vs 22.5 ± 17.6; p = 0.03; 16.5% increase from baseline in the telmisartan group). Conclusions. Telmisartan increased beta-cell function but did not decrease IMCL or liver fat content or other metabolic parameters among individuals with abdominal obesity.
Trial registration: ClinicalTrials.gov identifier: NCT00147264.
Introduction
Abdominal adiposity and ectopic lipid deposition in skeletal muscle and visceral organs like the liver or pancreas are implicated in the pathogenesis of insulin resistance and type 2 diabetes mellitus (Citation1). A significant body of evidence suggests that blockade of the renin–angiotensin system (RAS) with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II type 1-receptor (AT-1) blockers (ARBs) may reduce the incidence of type 2 diabetes (Citation2,Citation3). Based on our previous observations that angiotensin (Ang) II inhibits adipocyte differentiation via an AT-1 receptor-dependent mechanism (Citation4), we hypothesized that AT-1 receptor blockade would increase adipocyte differentiation, thereby diverting excess fat away from tissues such as skeletal muscle, liver and pancreas, thus reducing the risk for insulin resistance and subsequent diabetes (Citation5).
Telmisartan is a highly selective lipophilic AT-1 blocker, widely used for the treatment of hypertension (Citation6). Recent studies have demonstrated that telmisartan is also a partial peroxisome proliferation-activator receptor gamma (PPARg) agonist (Citation7). PPARg agonists like thiazolodinediones are a group of compounds that increase insulin sensitivity and reduce ectopic fat deposition and are effective in the prevention and treatment of type 2 diabetes mellitus (Citation8,Citation9). Data from numerous recent studies suggest that the AT-1 agonist telmisartan, either as a result of its AT-1 antagonistic and/or PPARg agonistic properties, may also have beneficial metabolic effects including improvements in glucose and lipid profiles (Citation10–14), adipokine levels (Citation15,Citation16) and/or fat distribution and body weight (Citation8).
The aim of the present study was to test the hypothesis that treatment with telmisartan will reduce intramyocellular lipid (IMCL) and hepatic fat storage, thereby improving insulin sensitivity as well as glucose and lipid metabolism in individuals with abdominal obesity.
Methods
Participants
We studied men and women aged between 30 and 70 years, with abdominal obesity (body mass index, BMI ≥ 30 kg/m2 and waist circumference > 102 cm in men and > 88 cm in women), with or without additional features of the metabolic syndrome as defined by the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (Citation17). Principal exclusion criteria included, previous treatment (prior 3 months) with or current indications (e.g. post-myocardial infarction, heart failure, proteinuria, etc.) for blockers of the RAS, type 2 diabetes mellitus, uncontrolled hypertension (SBP ≥ 160 mmHg and/or DBP ≥ 100 mmHg), serum creatinine > 130 μmol/l, unacceptable contraception (in women of child-bearing age), other relevant medical disorders and contraindications to magnetic resonance imaging (MRI). Participants were recruited through advertisement in local media and underwent a telephone screening, followed by a screening visit. The study protocol was approved by the local ethical committee and carried out in accordance with the Declaration of Helsinki as revised in 2000. All participants gave informed consent. The trial is publicly registered with ClinicalTrials.gov with the number NCT00147264.
Trial design
The 30-week prospective randomized study consisted of a 4–6-week single-blind placebo run-in period, during which participants received dietary counseling to adapt to a weight-maintaining diet containing 55% of energy as carbohydrate (CHO), 30% as fat (< 7% saturated) and 15% as protein (as documented in 3-day food records conducted throughout the study) (Citation18). Participants were also instructed to maintain their habitual level of physical activity. This was assessed with physical activity questionnaires and the use of an accelerometer (Actical® Physical Activity Monitor, Mini Mitter Co., Inc., Bend, OR, USA). After completion of the run-in period and following a baseline assessment, participants were randomized to telmisartan or placebo and a weight-maintaining low or high glycemic index diet, according to a two-by-two factorial design. Importantly both dietary interventions were isocaloric. Specifics of the low-glycemic dietary intervention and relevant outcomes will be reported separately. Following 4 weeks of once-daily telmisartan 80 mg or placebo, all participants were “force-titrated’ to telmisartan 160 mg or placebo for another 20 weeks. Participants were seen monthly during the randomization phase of the study, with the exception of a safety visit, scheduled 1-week after up-titration of telmisartan/placebo. Participants were instructed to take their medications in the morning after breakfast, except on the days of the clinic visits, on which the assigned drug was taken after completion of all investigations. Adverse events, intercurrent illnesses and participant compliance to the dosing regimen (assessed by counting the number of pills returned at the clinic visits) were monitored monthly.
Measurements
All of the measurements including biochemical and imaging were assessed in a blinded fashion, i.e. without knowledge of patient allocation. Biochemical measurements were performed after an overnight fast at baseline and after 24 weeks on treatment. Anthropometric measurements were taken in a standing position. BMI was calculated as the ratio of weight (kg) to height2 (m2). Waist circumference was measured with a tape measure placed horizontally around the abdomen at the level of the iliac crest at the end of a normal expiration, keeping the tape tense and parallel to the floor. Hip circumference was measured at the level of the major trochanter. Body composition was assessed by bioelectrical impedance analysis using a Bioscan 916 (Matlron International Ltd., Rayleigh, Essex, UK) as per manufacturer's instructions.
Intra-myocellular lipids were calculated by using single voxel proton MRS centered at the mid soleus muscle. Intrahepatocellular lipids were also obtained by using single voxel proton MRS localized to the right lobe of the liver. The intracellular lipids were calculated as the ratio of the area under the methylene peak (1.4) to that under the water peak (4.80)× 100. All magnetic resonance quantifications were performed on a Siemens 1.5T Symphony scanner. The coefficient of variation (CV) for IMCL determination in the soleus muscle is estimated to be 13.7% (Citation19). (Siemens AG, Munich, Germany).
Visceral (VAT) and subcutaneous adipose tissue (SAT) was assessed at L4. Computation of surface areas from the MRI scans was conducted using SliceOmatic 4.2 medical imaging software (SliceOmatic v.4.2, Tomovision, Montreal). VAT was defined as adipose tissue within the inside edge of the abdominal wall. SAT was defined as adipose tissue on the outside edge of the abdominal wall. The intra and inter-observer CVs for this method are 0.53% and 0.44% for SAT and 1.46% and 2.42% for VAT, respectively.
Blood pressure and heart rate were measured in the sitting participant using an automatic blood pressure monitor (BpTRU®, VSM MedTech Ltd., Vancouver, BC, Canada). Venous blood was collected for biochemical measurements after an overnight fast. Plasma glucose and lipid profile were measured using routine methods. Insulin was assayed on the Architect i1000 (chemiluminescent immunometric assay, intra-assay CV 7%). An oral glucose tolerance test (75 g glucose) was performed with measurement of glucose and insulin at − 15, 0, 30, 60 and 120 min. HOMA-IR (homeostasis model of assessment – insulin resistance) was calculated as: HOMA-IR = fasting insulin (uM/mL) × fasting glucose (mmol/l)/ 22.5 (Citation20). Insulinogenic index (IGI) was calculated as the ratio of the change in insulin to change in glucose from 0 to 30 min (Delta I30 /Delta G30) during the oral glucose tolerance test (OGTT) (Citation21). Total adiponectin levels were assayed by immunoassay using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). The intra- and interassay CVs were 4.1–4.8% and 7.1–9.8%, respectively.
Role of funding source
The study sponsors (Boehringer Ingelheim, Canada) were not involved in the trial design or conduct. All data were collected and analyzed by the investigators.
Statistics and data analyses
Assuming a two-sided alpha of 0.05, this study will have 90% power to detect a minimum difference in change in IMCL content between treatment groups of 3.7 and a standard deviation of 5, based on a t-test with 40 participants per group. Additional participants will be randomized to account for the anticipated drop-out rate of 30%.
Primary analysis was based on all randomized participants regardless of compliance with the protocol.
The primary objectives of this 2 × 2 factorial randomized controlled trial were to explore the effect of telmisartan treatment versus placebo in abdominally obese individuals consuming either a low or high glycemic diet, on IMCL content. The results of the LGI diet will be reported in a separate paper.
As the putative effect of AT1 blockade was expected to be independent of the putative effect of the LGI-diet, there was a scientific basis and rationale for studying both interventions in the same study population using a 2 × 2 factorial design.
No interaction between telmisartan and the LGI diet was anticipated. Therefore, all results will be summarized separately by the treatment margins, either as telmisartan vs placebo or LGI diet vs control diet and not by the factorial cells.
Tests for interaction between telmisartan and LGI diet will be done to identify any unexpected interaction effect and those with p < 0.01 will be considered suggestive of a possible effect.
An intention-to-treat analysis was completed. The primary endpoint was between group change in IMCL of the soleus muscle co-varying out baseline IMCL measurement, change in weight, BMI, and any other important covariate identified from the baseline characteristics.
Data analysis was performed by means of the SAS version 9.1 statistical package.
Results
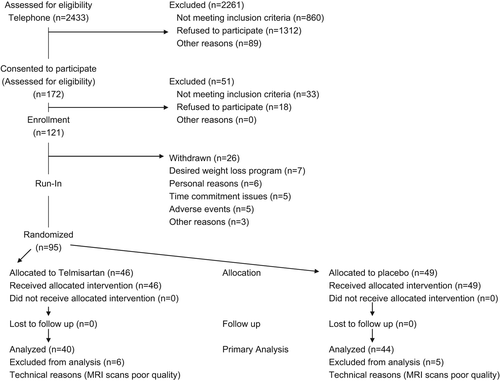
Telephone screening was conducted in 2433 potential participants, of whom 172 were invited for a screening visit (). Of the 121 eligible participants, who agreed to participate in the study, 95 completed the run-in phase and were subsequently randomized to telmisartan (n = 46) or placebo (n = 49). Forty-one participants in the telmisartan group and 47 participants in the placebo group completed the 24-week treatment period. Demographics and baseline characteristics were similar between the telmisartan and placebo groups ().
Table I. Demographic and clinical characteristics of participants at baseline.
Thirty nine percent of our participants had a low high-density lipoprotein (HDL)-cholesterol (< 1.3 mmol/l (males) < 1.0 mmol/l (women), 59% had triglycerides > 1.7mmol/l, 22% had BP > 140/90. The mean HOMA-IR at baseline was 3.61. Our lab uses a cut-off value of 2.6 for defining insulin resistance. Based on this value 60% of our participants at baseline were insulin resistant
The mean waist circumference for men and women at baseline were 106 cm and 121 cm respectively. At baseline IMCL content was positively correlated with liver fat (r2 = 0.23; p = 0.01), VAT/ SAT (r2 = 0.26; p = 0.03) and waist circumference (r2 = 0.34; p = 0.001).
After 24 weeks of treatment, participants treated with telmisartan had significantly lower systolic and diastolic blood pressures than controls (p < 0001) . The number of participants with impaired glucose tolerance both at baseline and end of the study, did not differ ().
Table II. Anthropometrics and body composition END- OF-STUDY telmisartan vs placebo difference in means controlling for baseline variables.
Table III. Blood pressure, heart rate and metabolic characteristics END-OF-STUDY telmisartan vs placebo difference in means controlling for baseline.
There were no significant treatment effects on IMCL content. (5.73 ± 1.11 vs 6.11 ± 1.11; p = 0.13), HOMA-IR (3.77 ± 2.12 vs 3.7 ± 2.12; p = 0.99), fasting plasma glucose (5.65 ± 0.54 vs 5.64 ± 0.54; p = 0.92), or the 120-min OGTT plasma glucose (8.4 ± 1.77 vs 8.43 ± 1.77; p = 0.94). Furthermore, there were no significant changes in body composition, VAT, VAT/SAT ratio or liver fat compared with baseline. Beta-cell function, as determined by the IGI, improved significantly (19.3 ± 13.7 vs 22.5 ± 17.6; p = 0.03; 16.5% increase from baseline in the telmisartan group). Total adiponectin increased marginally in the telmisartan group (48.9 ± 42.7 ng/ml vs 56.85 ± 37.6 ng/ml; p = 0.06).
The results did not change with adjustment of pre-specified baseline variables.
Discussion
Twenty-four weeks of treatment with telmisartan 160 mg in abdominally obese individuals had no discernible effect on IMCL, hepatic fat, body composition or lipid and glucose profiles. The rationale for the present trial was based on our previous observation that all components of the RAS are expressed in mature adipocytes (Citation22,Citation23) and that in vitro Ang II blocks the proliferation and differentiation of human preadipocytes (Citation4). Furthermore, in co-culture experiments, differentiation of preadipocytes was inhibited in the presence of mature adipocytes and this effect could be abolished by irbesartan, indicating that it was mediated by endogenous angiotensin II acting via type 1 receptors. Based on these observations, we hypothesized that in vivo inhibition of adipocyte development by Ang II would promote ectopic fat deposition thereby increasing insulin resistance and the subsequent risk for type 2 diabetes. This “lipotoxicity” hypothesis is supported by a number of indirect observations, such as the fact that surgical implantation of adipose tissue reverses diabetes in lipodystrophic mice (Citation24–26).
Further rationale for our use of telmisartan in our study was provided by the observation that telmisartan is distinct among ARBs because it exhibits PPARγ agonist activity, which is completely independent from its AT1R blocking properties (Citation27). Peroxisomal proliferator activator receptor-gamma (PPARγ) activation is also a modulator of preadipocyte differentiation (Citation28).
Our finding that telmisartan does not appear to have discernible effect on ectopic fat deposition or measure of glucose and lipid homeostasis are consistent with a recent study by Hsueh et al. (Citation29). This study examined the effect of telmisartan on 138 overweight/obese patients with components of the metabolic syndrome randomized to telmisartan or matching placebo for 16 weeks. In this study, telmisartan likewise had no effect on the IGI, calculated from oral glucose tolerance testing or on insulin resistance measured by hyperinsulinemic euglycemic clamp. As in our study, there were also no significant effects on lipid metabolism. However, unlike our study, most of the participants were normotensive obese subjects without evidence of insulin resistance.
Our findings are in line with a recently published large clinical trial. In the TRANSCEND Study (Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease) (Citation30), 21.8% of participants treated with telmisartan and 22.4% of those on placebo developed diabetes (relative ratio 0.95 [95% CI 0.83–1.10]; p = 0.51). Participants originally diagnosed with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) were equally likely to regress to normoglycemia (26.9 vs 24.5%) or to progress to incident diabetes (20.1 vs 21.1%; p = 0.59) on telmisartan or placebo. The investigators concluded that there was no evidence that addition of telmisartan prevents incident diabetes or leads to regression of IFG or IGT in individuals at high risk for cardiovascular disease. Importantly, new onset diabetes was not a primary outcome variable and this trial had very low power for detecting a 10% risk reduction (a level of risk reduction, which is in the range of the anti-diabetic effect that might be anticipated for an ARB). In contrast, in an Italian study, Fogari et al. (Citation31) compared the effect of telmisartan and eprosartan on insulin sensitivity in 50 overweight hypertensive patients. Insulin sensitivity, assessed by the glucose clamp technique, was significantly increased by telmisartan (2.25 ± 0.61 μmol/min/kg, p < 0.05 vs placebo) but not by eprosartan (0.25 ± 0.14 μmol/min/kg, p = ns). Similarly, if we included hypertensive individuals or individuals with a prior history of cardiovascular disease, we may have detected a positive outcome in the reduction of incident diabetes.
Although we did not see an effect of telmisartan on ectopic fat or insulin resistance, we observed a 16.5% improvement in beta-cell function (ISI improved from 19.3 ± 13.7 to 22.5 ± 17.6; p = 0.03), in the telmisartan treated group. This latter observation is in agreement with Negro et al. (Citation11) and Nagel et al. (Citation10), who observed a 32% and 35% improvement in the IGI respectively, indicating an improved beta-cell function. This finding may not be unexpected, as previous studies have confirmed that the RAS constituents, angiotensinogen, ACE and angiotensin II type 1 and 2 receptors (AT1R and AT2R), are present in pancreatic islets, specifically beta cells (Citation32) This has been postulated to inhibit insulin release in response to glucose exposure. Mechanistically, this could be mediated, in part, through alterations in islet pro-insulin synthesis and islet blood flow regulated by angiotensin II biosynthesis (Citation33).
We also observed a marginal increase in total adiponectin, which is a potent insulin sensitizer. This finding is consistent with other studies, which found significant increases in adiponectin levels with telmisartan (Citation12,Citation34). Two other studies (Citation14,Citation15), however, failed to detect an increase in adiponectin with telmisartan.
In a recent meta-analysis by Takagi & Umemoto (Citation35), telmisartan treatment reduced fasting insulin levels and improved insulin sensitivity over other ARB therapy. However, most randomized trials included in the present meta-analysis, were relatively small (< 100 participants) and did not report on clinical outcomes.
It should also be noted that a number of smaller studies have found a neutral (Citation26) or shown positive effects of telmisartan on gluco-metabolic response (Citation1,Citation10,Citation12,Citation13,Citation16). Importantly these previous studies were of short duration (Citation12,Citation13), with small sample sizes (Citation12,Citation13,Citation16) and were not randomized controlled trials (Citation12,Citation13).
Study strengths and limitations
We undertook a comprehensive investigation of the metabolic effects of Telmisartan in obese and overweight individuals. Importantly, in order to determine the direct effect of the intervention on IMCL content, we took considerable care to ensure that participant's weight and physical activity levels remained constant throughout the study. We also used a considerably higher dose of telmisartan than currently used in clinical practice and powered the study adequately to detect any clinically relevant metabolic effects.
The study may be limited in its duration (6 months), but there is nothing in the data to suggest that longer treatment would have elicited a different outcome of this study. Since this is an efficacy trial, generalizabilty of the results to other populations, e.g. hypertensive patients not consuming low glycemic or low fat diets (i.e. in the types of patients most commonly treated with telmisartan), may be limited.
In summary, the present study does not support the hypothesis that telmisartan treatment in abdominally obese, non-hypertensive individuals consuming either a low glycemic or low fat diet, reduces intramyocellular, hepatic or abdominal fat deposition nor that treatment with telmisartan has other clinically significant metabolic effects. Possible beneficial effects on insulin secretion may warrant further study.
Acknowledgements
We are grateful to Scott Lear and Simi Kohli, School of Kinesiology, Simon Fraser University, Burnaby, British Columbia, for assistance in the analyses of the MRI scans. The study was funded by Heart and Stroke Canada and Boehringer Ingelheim (BI), Canada (manufacturers of Telmisartan). BI were not involved in the trial design or conduct. All data were collected and analyzed by the investigators.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887.
- Scheen AJ. Prevention of type 2 diabetes mellitus through inhibition of the renin–angiotensin system. Drugs. 2004; 64:2537–2565.
- Ostergren J. Renin–angiotensin-system blockade in the prevention of diabetes. Diabetes Res Clin Pract. 2007;76 Suppl 1:S13–S21.
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707.
- Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–611.
- Sharpe M, Jarvis B, Goa KL. Telmisartan: A review of its use in hypertension. Drugs. 2001;61:1501–1529.
- Kurtz TW. Treating the metabolic syndrome: Telmisartan as a peroxisome proliferator-activated receptor-gamma activator. Acta Diabetol. 2005;42 Suppl 1:S9–S16.
- Yang X, Smith U. Adipose tissue distribution and risk of metabolic disease: Does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer?Diabetologia. 2007;50:1127–1139.
- , DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial InvestigatorsGerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–1105.
- Nagel JM, Tietz AB, Göke B, Parhofer KG. The effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjects. Metabolism. 2006;55: 1149–1154.
- Negro R, Formoso G, Hassan H. The effects of irbesartan and telmisartan on metabolic parameters and blood pressure in obese, insulin resistant, hypertensive patients. J Endocrinol Invest. 2006;29:957–961.
- Benndorf RA, Rudolph T, Appel D, Schwedhelm E, Maas R, Schulze F, et al. Telmisartan improves insulin sensitivity in nondiabetic patients with essential hypertension. Metabolism. 2006;55:1159–1164.
- Mori Y, Itoh Y, Tajima N. Telmisartan improves lipid metabolism and adiponectin production but does not affect glycemic control in hypertensive patients with type 2 diabetes. Adv Ther. 2007;24:146–153.
- Inoue T, Morooka T, Moroe K, Ikeda H, Node K. Effect of telmisartan on cholesterol levels in patients with hypertension – Saga Telmisartan Aggressive Research (STAR). Horm Metab Res. 2007;39:372–376.
- Makita S, Abiko A, Naganuma Y, Moriai Y, Nakamura M. Effects of telmisartan on adiponectin levels and body weight in hypertensive patients with glucose intolerance. Metabolism. 2008;57:1473–1478.
- Komiya N, Hirose H, Kawabe H, Itoh H, Saito I. Effects of telmisartan therapy on metabolic profiles and serum high molecular weight (HMW)-adiponectin level in Japanese male hypertensive subjects with abdominal obesity. J Atheroscler Thromb. 2009;16:137–142.
- National Cholesterol Education Program National Heart, Lung, and Blood Institute. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary, National Institutes of Health NIH Publication No. 01–3670, May. 2001.
- Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. Revision. 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association Circulation. 2000;102:2284.
- Li X, Youngren JF, Hyun B, Sakkas GK, Mulligan K, Majumdar S, et al. Technical evaluation of in vivo abdominal fat and IMCL quantification using MRI and MRSI at 3 T. Magn Reson Imaging. 2008;26:188–197.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419.
- Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta-cell function. Best Pract Res Clin Endocrinol Metab. 2003;17:305–322.
- Ailhaud G, Fukamizu A, Massiera F, Negrel R, Saint-Marc P, Teboul M. Angiotensinogen, angiotensin II, and adipose tissue development, Int J Obes Relat Metab Disord. 2000;24:S33–S35.
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707.
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes.1999;48:1113–1119.
- Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, et al. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–2343.
- Gavrilova O, Mareus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al.Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice, J Clin Invest. 2000;105:271–278.
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953.
- Jucker BM, Schaeffer TR, Haimbach RE, Mayer ME, Ohlstein DH, Smith SA, et al. Reduction of intramyocellular lipid following short-term rosiglitazone treatment in Zucker fatty rats: An in vivo nuclear magnetic resonance study. Metabolism. 2003;52:218–225.
- Hsueh W, Davidai G, Henry R, Mudaliar S. Telmisartan effects on insulin resistance in obese or overweight adults without diabetes or hypertension. J Clin Hypertens. 2010;12:746–752.
- Barzilay JI, Gao P, Rydén L, Schumacher H, Probstfield J, Commerford P, et al.; TRANSCEND Investigators. Effects of telmisartan on glucose levels in people at high risk for cardiovascular disease but free from diabetes: The TRANSCEND study. Diabetes Care. 2011;34:1902–1907.
- Fogari R, Zoppi A, Ferrari I, Mugellini A, Preti P, Lazzari P, et al. Comparative effects of telmisartan and eprosartan on insulin sensitivity in the treatment of overweight hypertensive patients. Horm Metab Res. 2009;41:893–898.
- Lua T, Carlsson PO, Leung PS. Evidence of a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–248.
- Bonora E. Protection of pancreatic beta-cells: Is it feasible?Nutr Metab Cardiovasc Dis. 2008;18:74–83.
- Furuhashi M, Ura N, Higashiura K, Murakami H, Shimamoto K. Blockade of the renin–angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81.
- Takagi H, Umemoto T. Telmisartan improves insulin sensitivity: A meta-analysis of randomized head-to-head trials. Int J Cardiol. 2012;156:92–96.