Abstract
Aim. To assess prevalence, type and covariates of abnormal left ventricular (LV) geometry in untreated native Tanzanian patients with hypertension in relation to normotensive controls. Methods. Echocardiography was performed in 161 untreated hypertensive outpatients and 80 normotensive controls at a tertiary hospital in Tanzania. Hypertensive heart disease was defined as presence of increased LV mass or relative wall thickness (RWT). Results. The prevalence of hypertensive heart disease increased with the severity of hypertension and was on average 62.1% among patients and 12.5% in controls. In multivariate analyses, higher LV mass index was associated with higher systolic blood pressure (β = 0.28), body mass index (β = 0.20), peak early transmitral to medial mitral annulus velocity ratio (β = 0.16), and with lower stress-corrected midwall shortening (scMWS) (β = − 0.44) and estimated glomerular filtration rate (β = − 0.16), all p < 0.05. Higher RWT was associated with higher systolic blood pressure (β = 0.16), longer E-wave deceleration time (β = 0.23) and lower scMWS (β = − 0.66), irrespective of LV mass (all p < 0.05). Conclusion. Subclinical hypertensive heart disease is highly prevalent in untreated native hypertensive Tanzanians and associated with both systolic and diastolic LV dysfunction. Management of hypertension in Africans should include high focus on subclinical hypertensive heart disease.
Introduction
Despite the increase in non-communicable diseases in Africa (Citation1), in particular hypertension and its cardiac and renal complications, studies on hypertensive heart disease among native Africans have so far been limited. Abnormal left ventricular (LV) geometry including LV hypertrophy and increased relative wall thickness (RWT) without LV hypertrophy (i.e. concentric LV remodelling) are well-known indicators of hypertensive heart disease and increased cardiovascular morbidity and mortality, both in Caucasian hypertensive and general population (Citation2–5). Studies in hypertension in African Americans have shown higher prevalence of abnormal LV geometry (Citation6–8), and higher incidence of cardiovascular complications compared with Caucasians (Citation9,Citation10). However, few data from studies on untreated hypertensive subjects in sub-Saharan Africa have been published, and few studies have included healthy control subjects (Citation11–14). Given the increasing immigration of Africans to Europe, knowledge of hypertensive heart disease in Africans is of interest to African as well as European health care providers. Thus the aim of the present study was to give new knowledge on prevalence, type and covariates of abnormal LV geometry in native untreated Tanzanian patients with hypertension in relation to normotensive controls.
Materials and methods
Patient population
This prospective study was designed to include all untreated hypertensive patients presenting as outpatients at the Department of Medicine Muhimbili National Hospital in Dar es Salaam, Tanzania, a tertiary referral hospital, between September 2009 and May 2010. Patients were eligible if they were ≥ 18 years of age, confirmed hypertensive (blood pressure ≥ 140/90 mmHg on two different occasions) and were never-treated. Exclusion criteria were previous history of cardiovascular disease (self-reported), history of diabetes mellitus, pregnancy-induced hypertension, symptomatic rheumatic valvular heart disease or uraemia.
In total 200 patients with untreated hypertension were referred from local hospitals in Dar es Salaam without cardiology service during the study period. Of these, 10 patients were excluded because they also had diabetes, 23 patients did not show up for echocardiography and six patients were excluded due to symptomatic end stage renal disease. The remaining 161 (80.5%) patients (aged 20–79 years) constitute the present study population.
For comparison, 80 normotensive healthy adults were recruited as controls among hospital employees, family members escorting patients as well as prospective kidney donors. Control subjects were required to be in the same age and gender distribution as patients (with a patient to control ratio of 2:1), and without known disease of any kind, not using any type of medication and have documented blood pressure < 140/90 mmHg on two or more occasions.
The study was performed in accordance with the Helsinki declaration and received ethical approval from the Muhimbili University of Health's research and publication committee board, and all participants signed a written consent form.
Clinical assessment and laboratory tests
A structured questionnaire was used to collect information on socio-demographic characteristics and cardiovascular risk factors. Height and weight were measured and used to calculate body mass index (BMI). Obesity was defined as BMI ≥ 30 kg/m2 (Citation15). Waist circumference was measured at the level of the umbilicus and was used as a measure of central obesity. Sitting blood pressure was taken by an experienced nurse using a mercury sphygmomanometer, in accordance with the Joint European Society of Hypertension and European Society of Cardiology guidelines on hypertension management (Citation16). Supine blood pressure measured at completion of the echocardiogram was used for calculations of haemodynamic variables.
Fasting blood samples were collected and analysed for glucose, cholesterol and renal function. Estimated glomerular filtration rate (eGFR) was calculated using the Cockcroft–Gault equation (Citation17). A first morning spot urine sample was collected and tested for urine albumin creatinine ratio (UACR). Abnormal albuminuria was defined as UACR > 30 mg/g (Citation18). UACR was not tested among the healthy controls.
Echocardiography
All echocardiograms were done by the same licensed cardiologist (PC) using a SONOS 7500 Phillips echocardiograph with 3-MHz transducer. Patients were examined in left lateral decubitus position following a standardized previously published protocol (Citation19). All images were stored on high definition VHS tapes and magnetic optical disks and transferred to a Tomtec work station equipped with Image Arena version 4.1 software (TomTec Imaging Systems GmbH, Unterschielssheim, Germany), for digital analysis at the Echocardiography Research Laboratory at Haukeland University Hospital in Bergen, Norway. All studies were first analysed by the primary investigator (PC) and then proof read by a highly experienced reader (EG).
Quantitative echocardiography was performed following the joint European Association of Echocardiography and American Society of Echocardiography guidelines (Citation20). LV hypertrophy was considered present when LV mass indexed for height2.7 (LVMI) exceeded the prognostically validated cut-off values of 46.7 g/m2.7 in women and 49.2 g/m2.7 in men (Citation21). RWT was calculated as the posterior wall thickness/LV internal radius ratio at end diastole and considered increased if > 0.42. Patients were then categorized into four LV geometric patterns based upon LVMI and RWT in combination (Citation20). Patients with normal LVMI were defined as normal LV geometry if RWT was normal and concentric remodelling if RWT was increased. Patients with LV hypertrophy were defined as eccentric LV hypertrophy if RWT was normal and as concentric LV hypertrophy if also RWT was increased.
Stroke volume and LV ejection fraction were calculated using biplane Simpson's method and LV ejection fraction was considered low when < 55% (Citation20). Systolic function was also assessed from midwall shortening (MWS) calculated using a previously validated equation, taking into consideration the epicardial migration of the midwall during systole and adjusted for circumferential end-systolic stress (Citation22,Citation23). Stress-corrected MWS (scMWS) was considered low if < 87% in men and < 90% in women (Citation24).
LV filling was recorded with pulsed wave Doppler sampling between the mitral leaflets tips in apical four-chamber views. The early (E) and atrial (A) waves were traced to derive peak velocities, E-wave deceleration time and E/A ratio. Isovolumic relaxation time was measured from the leading edge of the aortic valve closure spike to the leading edge of the mitral valve opening spike in apical five-chamber views. Early diastolic septal mitral annular plane velocity (E’) was measured by spectral tissue Doppler in apical four-chamber views. The ratio of E to E’ velocity (E/E’ ratio) was taken as an estimation of LV filling pressure (Citation25) and diastolic dysfunction was defined as E/E’≥ 15 (Citation26).
Statistical methods
Data management and statistical analysis was performed using IBM SPSS for Windows version 19 (IBM SPSS, Chicago, IL). Data is presented as mean ± standard deviation for continuous variables and percentages for categorical variables. Groups of patients were compared using χ2 test for categorical variables and unpaired Student's t-test or one-way ANOVA with Sheffe's post hoc test for continuous variables, as appropriate. UACR was not normally distributed and therefore log-transformed before included in uni- and multivariate analyses. Bivariate correlations were assessed by Pearson's correlation coefficient and the independent covariates of higher LVMI and RWT were identified in multivariate linear regression analysis, run with an enter procedure and collinearity statistics. Reproducibility of measurement of LV mass was tested in a subset of 50 patients by the intraclass correlation coefficient with 95% confidence interval (CI). A two-tailed p-value of ≤ 0.05 was considered statistically significant.
Results
Patients’ characteristics
The hypertensive patients were on average 5.9 years older than normotensive controls (p < 0.01), as a consequence of inability to find healthy controls in the highest age range, but were otherwise well matched for gender (). Patients had significantly higher systolic and diastolic BP than controls by design, but also higher BMI and lower creatinine clearance ().
Table I. Clinical and demographic characteristics of the study population.
LV geometry and function
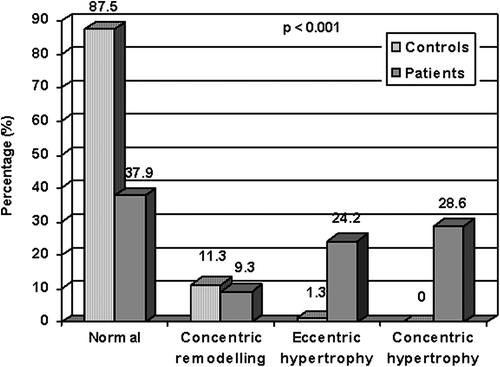
LV geometry differed significantly between patients and controls (, ). In total, 62.1% of the patients had abnormal LV geometry, predominantly LV hypertrophy (24.2% eccentric and 28.6% concentric LV hypertrophy). In contrast, abnormal LV geometry was found in 12.5% of controls, in all but one person of concentric remodelling type (). LV systolic function was lower and measures of LV diastolic function were more unfavourable in patients when compared with controls (). Measurement of LV mass showed excellent reproducibility with an intraclass correlation coefficient of 0.92 (95% CI 0.88–0.96).
Table II. Echocardiographic findings in patients and controls.
In univariate analyses, higher LVMI and RWT were both associated with higher age, blood pressure, serum creatinine and E/E’ ratio and with lower eGFR and scMWS in the total study population (, ). In addition, higher LVMI was also associated with lower LV ejection fraction as well as higher BMI (). Among patients only, higher LVMI was associated with higher logUACR (r = 0.31, p < 0.001) while no significant association was found between higher RWT and logUACR (r = 0.12, p = 0.15). In the patient group, prevalences of abnormal LV geometry and abnormal albuminuria both increased with increasing severity of hypertension (). Combined abnormal LV geometry and abnormal albuminuria was found in 13.6%, 20.4% and 40% of patients with grade I, grade II and grade III hypertension respectively (p < 0.01) (). Low scMWS was found in 55.3% of patients, and patients with reduced scMWS were more likely to have combined abnormal LV geometry and abnormal albuminuria when compared with patients with normal scMWS (35.9% vs 8.6%, p < 0.001).
Figure 2. Higher left ventricular mass index (LVMI) was associated with lower stress-corrected midwall shortening (scMWS) (left panel) and higher peak early transmitral blood velocity to medial mitral annulus velocity (E/E’) ratio (right panel).
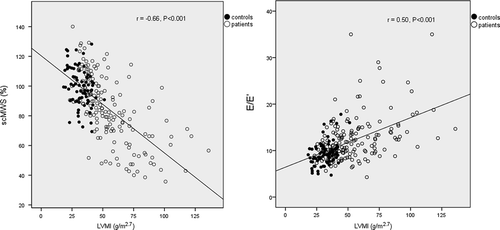
Figure 3. Prevalence of abnormal left ventricular (LV) geometry (solid bars), abnormal albuminuria (lined bars) and combined abnormal LV geometry and abnormal albuminuria (dotted bars) in groups of patients with grade I, grade II and grade III hypertension. (p = 0.001, p = 0.03 and p = 0.008 for the differences in prevalences of abnormal LV geometry, abnormal albuminuria and both, respectively).
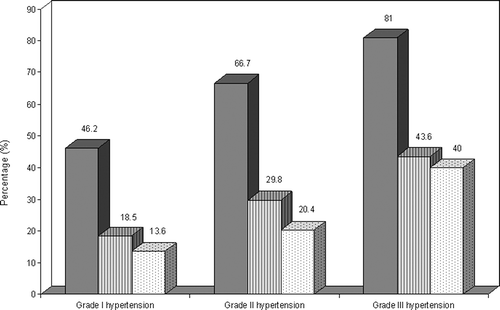
Table III. Correlates of higher LVMI and RWT in the total population.
In multiple linear regression analyses in the total population, higher LVMI was independently associated with higher systolic blood pressure, BMI and E/E’ ratio and with lower scMWS and eGFR (multiple R2 = 0.60, p < 0.001) (), while the independent covariates of higher RWT (i.e. concentric LV geometry) were higher systolic blood pressure, longer E-wave deceleration time and lower scMWS (multiple R2 = 0.55, p < 0.001) (). When the multivariate models were run within the patients group only, similar results were found, except that also higher BMI was identified as an additional independent covariate of higher RWT (). Substituting eGFR with presence of abnormal albuminuria in the model for LVMI (including only patients), yielded similar results and identified abnormal albuminuria as an independent covariate (presence of abnormal albuminuria, β = 0.17, p < 0.01).
Table IV. Independent predictors of higher LVMI and RWT identified in multivariate linear regression analyses.
Discussion
Hypertension and its cardiovascular complications are on the rise in Africa (Citation1). Given the increasing immigration of Africans to Europe, knowledge of subclinical cardiac complications in hypertensive Africans is relevant for European doctors managing hypertension. The present study reports new knowledge by demonstrating that never-treated hypertensive patients referred to a tertiary hospital in Tanzania have five times higher prevalence of abnormal LV geometry than found among healthy controls. In particular, LV hypertrophy was present in 53% of never-treated hypertensive patients as opposed to 1% in the controls. As seen in the multivariate analyses, systolic blood pressure was the primary determinant of subclinical hypertensive heart disease (i.e. LV hypertrophy and concentric LV geometry) in the present study. Furthermore, the prevalence of subclinical hypertensive heart disease increased in a graded fashion with increasing severity of hypertension.
Our finding that abnormal LV geometry is highly prevalent among native Tanzanian hypertensive patients extends findings from previous studies in hypertensive African Americans as well as native African hypertensive populations (Citation12–14,Citation27). In a study by Aje et al. (Citation12) among 100 newly diagnosed hypertensive Nigerians, abnormal LV geometry was found in 72%, slightly higher than our prevalence of 62%. The difference in LV geometry was mainly due to a higher prevalence of concentric remodelling in their study, while LV hypertrophy was higher in the present study (53% vs 46%). The prevalence of LV hypertrophy found in the present study is, however, lower than the 71% prevalence reported from a recent study in black hypertensive patients in South Africa by Libhaber et al. (Citation14), probably reflecting the higher prevalence of obesity in their study. Of note, Libhaber et al. found that > 50% of the patients had residual LV hypertrophy after 1 year despite antihypertensive treatment (Citation14). It was recently pointed out in a publication from the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study that regression of LV hypertrophy is blunted in hypertension if obesity coexists (Citation28). In the present study, obesity was found in 37% of the patients and higher BMI predicted higher LVMI independent of systolic blood pressure, in keeping with previous studies in hypertensive as well as general population (Citation8,Citation29–32). These findings further emphasise the need to control overweight and obesity in patients with hypertension.
Myocardial function has not been reported from previous studies in native African hypertension. As demonstrated by our findings, both higher LVMI and higher RWT were independently associated with reduced systolic myocardial function in multivariate analyses. In particular, patients with reduced myocardial function were more likely to have combined abnormal LV geometry and abnormal albuminuria. We recently also reported the association between concentric LV geometry and reduced myocardial function in a study of Tanzanian type 2 diabetic patients (Citation19). Furthermore, among hypertensive patients participating in the Hypertension Genetic Epidemiology Network (HyperGEN) study, LV myocardial function was reported to be lower in Africans compared with Caucasians (Citation6). We did not include LV myocardial deformation analysis in the present study and the prevalence of subclinical systolic dysfunction may therefore have been underestimated. However, low myocardial function was noted in more than half of the patients and low LV ejection fraction in one in five, probably reflecting longstanding hypertension, but possibly also high susceptibility among hypertensive Sub-Saharan Africans for development of cardiac dysfunction.
Both concentric LV geometry and LV hypertrophy were also associated with impaired LV diastolic function, reflected by increased E/E’ ratio and longer E-wave deceleration time in the present study, extending previous findings from the HyperGEN study as well as the Atherosclerosis Risk In Communities (ARIC) study into this sub-Saharan African population (Citation27,Citation33). Both concentric LV geometry and LV hypertrophy are associated with unfavourable metabolic abnormalities influencing cellular mechanisms of active relaxation as well as abnormalities of coronary microcirculation and fibrosis-related extracellular matrix disarray, which may all explain myocardial dysfunction in such patients (Citation34).
The association between abnormal albuminuria and abnormal LV geometry in essential hypertension is well established (Citation35,Citation36) and microalbuminuria is a known independent predictor of cardiovascular morbidity and mortality in hypertensive patients (Citation37). In the present study, the prevalence of abnormal albuminuria increased with the severity of hypertension, consistent with the findings in untreated African American hypertensive patients in the ARIC study (Citation38). Furthermore, we found that abnormal albuminuria independently predicted higher LV mass, in line with findings in the LIFE study (Citation36). Although parallel cardiac and renal target organ damage may be found in essential hypertension (Citation36), abnormal LV geometry is also commonly found in hypertensive African patients without abnormal albuminuria, as demonstrated by our findings.
Although the selectivity and limited number of study participants limit generalizability of our results, the strength of this study is the systematic prospective data collected from never-treated hypertensive patients and inclusion of a healthy control group. Despite including some hospital workers in the control group, the study results permit a better understanding of the prevalence and covariates of abnormal LV geometry in native, sub-Saharan hypertensive patients. We believe that this documentation is important and highly relevant for awareness of cardiac consequences of lack of diagnosis and treatment of the most common non-communicable disease in Africans: hypertension.
In conclusion, subclinical hypertensive heart disease was highly prevalent among never-treated Tanzanian patients with hypertension. Prevalence of subclinical hypertensive heart disease increased with the severity of hypertension, and was associated with reduced LV systolic and diastolic function.
Disclosure: The authors report no conflicts of interest.
References
- Hendriks ME, Wit FW, Roos MT, Brewster LM, Akande TM, de Beer IH, et al. Hypertension in sub-Saharan Africa: Cross-sectional surveys in four rural and urban communities. PLoS One. 2012;7:e32638.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566.
- Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlof B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study). Eur J Echocardiogr. 2008;9:809–815.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995; 25:871–878.
- Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–738.
- Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, et al. Differences in left ventricular structure between black and white hypertensive adults: The Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: The Dallas Heart Study. Hypertension. 2005;46:124–129.
- Gottdiener JS, Reda DJ, Materson BJ, Massie BM, Notargiacomo A, Hamburger RJ, et al. Importance of obesity, race and age to the cardiac structural and functional effects of hypertension. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1994;24:1492–1498.
- Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich). 2003;5:5–11.
- Fox ER, Alnabhan N, Penman AD, Butler KR, Taylor HA, Jr., Skelton TN, et al. Echocardiographic left ventricular mass index predicts incident stroke in African Americans: Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2007; 38:2686–2691.
- Ogah OS, Bamgboye AE. Correlates of left ventricular mass in hypertensive Nigerians: An echocardiographic study. Cardiovasc J Afr. 2010;21:79–85.
- Aje A, Adebiyi AA, Oladapo OO, Dada A, Ogah OS, Ojji DB, et al. Left ventricular geometric patterns in newly presenting Nigerian hypertensives: An echocardiographic study. BMC Cardiovasc Disord. 2006;6:4.
- Akintunde A, Akinwusi O, Opadijo G. Left ventricular hypertrophy, geometric patterns and clinical correlates among treated hypertensive Nigerians. Pan Afr Med J. 2010;4.
- Libhaber E, Norton G, Libhaber C, Woodiwiss A, Candy G, Essop M, et al. Prevalence of residual left ventricular structural changes after one year of antihypertensive treatment in patients of African descent: Role of 24-hour pulse pressure. Cardiovasc J Afr. 2012;23:1–6.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults – The evidence report. National Institutes of Health. Obes Res. 1998;6: 51S–209S.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Jensen JS, Clausen P, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant. 1997;12:6–9.
- Chillo P, Lwakatare J, Lutale H, Gerdts E. Increased relative wall thickness is a marker of subclinical cardiac target-organ damage in African diabetic patients. Cardiovasc J Afr. 2012; 23:1–7.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
- de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062.
- de Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, et al. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23: 1444–1451.
- Gaasch WH, Zile MR, Hoshino PK, Apstein CS, Blaustein AS. Stress-shortening relations and myocardial blood flow in compensated and failing canine hearts with pressure-overload hypertrophy. Circulation. 1989;79:872–883.
- Bella JN, Palmieri V, Roman MJ, Paranicas MF, Welty TK, Lee ET, et al. Gender differences in left ventricular systolic function in American Indians (from the Strong Heart Study). Am J Cardiol. 2006;98:834–837.
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30: 1527–1533.
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102: 1788–1794.
- Fox ER, Taylor J, Taylor H, Han H, Samdarshi T, Arnett D, et al. Left ventricular geometric patterns in the Jackson cohort of the Atherosclerotic Risk in Communities (ARIC) Study: Clinical correlates and influences on systolic and diastolic dysfunction. Am Heart J. 2007;153:238–244.
- Gerdts E, de Simone G, Lund BP, Okin PM, Wachtell K, Boman K, et al. Impact of overweight and obesity on cardiac benefit of antihypertensive treatment. Nutr Metab Cardiovasc Dis. 2013;23:122–129.
- de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, et al. Association of left ventricular hypertrophy with metabolic risk factors: The HyperGEN study. J Hypertens. 2002;20:323–331.
- Fox E, Taylor H, Andrew M, Han H, Mohamed E, Garrison R, et al. Body mass index and blood pressure influences on left ventricular mass and geometry in African Americans: The Atherosclerotic Risk In Communities (ARIC) Study. Hypertension. 2004;44:55–60.
- Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, et al. Longitudinal tracking of left ventricular mass over the adult life course: Clinical correlates of short- and long-term change in the Framingham offspring study. Circulation. 2009;119:3085–3092.
- Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008;21:1144–1151.
- de Simone G, Kitzman DW, Chinali M, Oberman A, Hopkins PN, Rao DC, et al. Left ventricular concentric geometry is associated with impaired relaxation in hypertension: The HyperGEN study. Eur Heart J. 2005;26: 1039–1045.
- Brilla CG, Maisch B, Weber KT. Myocardial collagen matrix remodelling in arterial hypertension. Eur Heart J. 1992;13: 24–32.
- Djousse L, Kochar J, Hunt SC, North KE, Gu CC, Tang W, et al. Relation of albuminuria to left ventricular mass (from the HyperGEN Study). Am J Cardiol. 2008;101:212–216.
- Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlof B, et al. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J. 2002;143:319–326.
- Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy?. The LIFE study. Diabetes Care. 2006;29:595–600.
- Hsu CC, Brancati FL, Astor BC, Kao WH, Steffes MW, Folsom AR, et al. Blood pressure, atherosclerosis, and albuminuria in 10,113 participants in the atherosclerosis risk in communities study. J Hypertens. 2009;27:397–409.