Abstract
The influence of chronological ageing on the components of the cardiovascular system is of fundamental importance for understanding how hemodynamics change and the cardiovascular risk increases with age, the most important risk marker. An increase in peripheral vascular resistance associated with increased stiffness of central elastic arteries represents hallmarks of this ageing effect on the vasculature, referred to as early vascular ageing (EVA). In clinical practice, it translates into increased brachial and central systolic blood pressure and corresponding pulse pressure in subjects above 50 years of age, as well as increased carotid–femoral pulse wave velocity (c-f PWV) – a marker of arterial stiffness. A c-f PWV value ≥ 10 m/s is threshold for increased risk according. Improved lifestyle and control of risk factors via appropriate drug therapy are of importance in providing vascular protection related to EVA. One target group might be members of risk families including subjects with early onset cardiovascular disease.
Introduction
Ageing represents an important risk marker in the development of cardiovascular disease (CVD). Nevertheless, the question arises: how immutable is chronological age as a risk factor? Can we make a distinction between physiologic or successful arterial ageing, which may be immutable, and early, accelerated or pathologic arterial aging, which is not? (Citation1) Pathologic ageing often starts in adolescence, while CVD events may not appear until upper middle age or beyond. Thus, there is a potential opportunity to attenuate the process of pathological ageing with early and effective lifestyle and/or therapeutic drug intervention.
This overview will be limited to brachial and central blood pressure (BP) and pulse wave velocity (PWV) as metrics for evaluating physiologic and pathologic arterial ageing. In particular, the focus will be on the value of: (i) mean arterial pressure (MAP) and elevated diastolic BP (DBP) as risk markers of peripheral vascular resistance (PVR); (ii) brachial and central pulse pressure (PP) as a risk marker of arterial stiffness; (iii) PWV as the “gold standard” for measuring arterial stiffness; (iv) arterial stiffness and PVR as contributors to pathologic arterial ageing; and (v) early and effective risk factor control for minimizing arterial ageing and preventing or delaying future CVD events. These are some of the themes that will be explored, using data from collaborative investigations of the Framingham Heart Study and population-based studies in Sweden, as well as from the more recent literature on pathologic arterial ageing, or as it is sometimes called, early vascular ageing (EVA) (Citation2–4).
Basics regarding BP measurement and interpretation
Accurate and precise office and clinic recording of BP have been limited in the past by both random and systematic errors of measurement (Citation5). However, there has been considerable improvement in the reliability of BP data with innovations that include: (i) the use of the oscillometric method for eliminating terminal digit preference and (ii) the use of home BP recordings to supplement office or clinic reading in order to increase sample size and identify white coat and masked hypertension (Citation5). With these innovations and the occasional use of 24-h BP monitoring, both random and systematic errors have been minimized and BP measurements show improved correlation with target organ damage and CVD endpoints.
Rather than defining hypertension as a value of ≥ 140 systolic BP (SBP) and/or ≥ 90 DBP, there is evidence of a continuous, graded level of BP that correlates with CVD mortality and morbidity in observational studies (Citation6). Indeed, the Prospective Studies Collaboration noted that observational BP levels was related to ischemic heart disease and stroke mortality without any threshold down to values as low as 115/75 mmHg (Citation7). For persons 40–69 years of age, there was a doubling of ischemic heart disease and stroke mortality for every 20-mmHg increment in SBP and 10-mmHg increment in DBP throughout the entire range of BP (Citation7).
The term “essential hypertension” was coined many years ago because it was thought essential that BP rises with ageing in order to compensate for atherosclerotic vessels. Paul Dudley White, in his 1931 Textbook of Cardiology, wrote: “Hypertension may be an important compensatory mechanism which should not be tampered with, even were it certain that we could control it.” However, with the introduction of effective antihypertensive agents in the second half of the 20th century, it soon became evident that effective lowering of BP by drug therapy resulted in a decrease in CVD morbidity and mortality (Citation8). Thus, rather than being a risk marker, the rise in BP represents a true and treatable risk factor for CVD events.
Pathways in the development of pathological arterial aging
A number of known and emerging biomarkers that are associated with pathologic ageing are listed in . Because most of these biomarkers come from cross-sectional studies, it is difficult to determine whether these processes coexist in any given individual and to establish causative pathways between these entities. Of this long list of possible biomarkers of arterial ageing, this presentation will focus only on hemodynamics of elevated BP – specifically on PVR and large artery stiffness.
Table I. Biomarkers of pathologic arterial ageing.
The principal components of BP consist of both a steady component (MAP) and a pulsatile component, PP. Major components of MAP are ventricular ejection and PVR. The major components of PP (the difference between SBP and DBP) are ventricular ejection, large artery stiffness and wave reflection.
PVR is determined by vessels < 300 μm in diameter, which include pre-capillary arterioles and small arteries (Citation9). Associated with hypertension, the vessels of the microcirculation can increase resistance in three ways. First, humoral and autonomic nervous system alterations in vasomotor tone and inherent myogenic tone can enhance vasoconstriction or reduce vasodilator responses. Second, there may be structural alternations that increase the wall-to-lumen ratio of pre-capillary resistance vessels (remodeling and hypertrophy). Lastly, there may be reversible or non-reversible rarefaction (reduction in the density) of arterioles and capillaries within a given vascular bed. Any one or a combination of two or three of these mechanisms can increase PVR and lead to impaired tissue perfusion, ischemia and eventual target organ damage (Citation10). The MAP equation is a “proxy” measure of PVR. MAP, in mmHg, is defined by (1/3 SBP)+(2/3 DBP). Therefore, increased DBP is always associated with increased PVR, but a normal or reduced DBP can be associated with increase PVR when there are large increases in SBP. In general, PVR is increased in proportion to the height of BP and has been considered the cardinal hemodynamic manifestation of essential hypertension.
Pulse pressure and arterial stiffness
The association between arterial stiffening, the rise in brachial PP and ageing has been well described in populations worldwide. PP represents the pulsatile or dynamic component of the circulation during systole. The key point to remember is that DBP rises with increased systemic PVR but falls with increased arterial stiffness. Brachial PP represents a surrogate measurement of central elastic artery stiffness – the thoracic aorta and its immediate branches – in the presence of a constant cardiac output and heart rate. Thus, the central arterial stiffening is manifested by three factors: (i) a rise in PP leading to (ii) a rise in SBP and (iii) a fall in DBP.
Central arterial elasticity is critically dependent on normal content and function of the matrix protein elastin, which, with a half-life of 40 years, is one of the most stable proteins in the body (Citation11). Despite this stability, fatigue of elastin fibers and lamellae can occur by the sixth decade of life from the accumulated cyclic stress of more than two billion aorta expansions during ventricular contraction across the lifespan. Long-standing cyclic stress in the media of elastin-containing arteries produces fatigue and eventual fracturing and disarray of elastin along with structural changes of the extracellular matrix that include proliferation of collagen and deposition of calcium. Humoral factors, cytokines and oxidative metabolites may also play a role (Citation11). The process, classically termed arteriosclerosis, results in increased stiffness of the aortic wall.
Importantly, not all arteries become stiff with age. Whereas long-term structural changes with ageing cause increased stiffness of the thoracic aorta and its branches (elastic arteries), the more peripheral muscular arteries (such as the brachial and femoral artery) retain their normal properties or may even become less stiff in people with hypertension.
To summarize, in the absence of changes in cardiac output and stroke volume, SBP rises with both increased PVR and increased arterial stiffness; DBP rises with increased PVR and falls with increased arterial stiffness; but MAP rises only with increased PVR; and PP rises only with increased arterial stiffness.
Why is BP a good metric for vascular ageing?
Participants in the Framingham Heart Study were noted to have elevated BP without additional CVD risk factors < 20% of the time, whereas there was greater than a 50% chance of having two, three or even four additional CVD risk factors associated with elevated BP (Citation12). Thus CV risk factors tended to cluster with elevated BP in numbers greater than by chance alone. These treatable cardiometabolic risk factors, most often in association with high-normal BP or hypertension, consist of abdominal obesity, elevated serum triglycerides, decreased high-density lipoprotein (HDL)-cholesterol, impaired fasting glucose or frank diabetes – contributing to the so-called “metabolic syndrome” when multiple abnormalities are present in an individual. Indeed, it has been shown that abdominal obesity and the metabolic syndrome are independently associated with increased PVR and increased aortic stiffness, both of which are associated with pathologic ageing (Citation13,Citation14). Other CVD risk factors are increased low-density lipoprotein (LDL)-cholesterol, cigarette smoking and physical inactivity. The increase in arterial stiffness and pathologic ageing leading to EVA is not only a reflection of elevated BP, but also of the global risk that includes all associated CVD risk factors. Similarly, other disease processes such as diabetes, chronic kidney disease and generalized atherosclerosis can increase stiffening of central elastic arteries, and therefore, accelerate pathologic arterial ageing or EVA (Citation2–4). The EVA concept has been more discussed during recent years but is still a concept not well defined or understood. It cannot be regarded as a syndrome on its own but merely represents biological associations (Citation4). A central component is arterial stiffness (arteriosclerosis) linked to hemodynamic changes, but there are also a number of non-hemodynamic components of EVA such as hyperglycemia, lipid disturbances and chronic inflammation. This has been debated at two international conferences on EVA held in Portugal in 2011 and 2012. Further data on the non-hemodynamic components of EVA will be presented at the European Society of Hypertension Meeting in Milan, June 2013.
Although arteriosclerosis is often confused with atherosclerosis, these two disease states are independent but frequently occur together in the same individual. Atherosclerosis is primarily focal by nature, starts in the intima and tends to be occlusive. By contrast, arteriosclerosis tends to be diffuse, starts in the media, and frequently results in a dilated and tortuous aorta, especially the proximal aortic root. Moreover, the pathophysiology of atherosclerosis is that of inflammatory disease with lipid-containing plaques and predominantly downstream ischemic disease. In contrast, arteriosclerosis represents degenerative arterial disease, which results in predominantly upstream increased thoracic aortic stiffness and elevated left ventricular load. Therefore, both arteriosclerosis and atherosclerosis, although separate conditions, predispose to progressive arterial stiffness and pathologic ageing that ultimately can result in adverse CVD events.
Hemodynamic patterns of age-related changes in BP
Both cross-sectional and longitudinal population studies show that SBP levels rise progressively beginning in adolescence (Citation15,Citation16). In contrast, DBP initially increases during adolescence and young adulthood, levels off at approximately age 50 years, and decreases after age 60 years. Thus, PP begins to increase after age 50. The rise in SBP and DBP up to age 50 years can best be explained hemodynamically by the dominance of increasing PVR (indicated by an increasing MAP and DBP) over arterial stiffness (). The transition at an age of 50–60 years when DBP levels off constitutes a near balancing of increased resistance and arterial stiffness. After age 60 years, the fall in DBP and the rapid widening of brachial PP become surrogate indicators of arterial stiffening. As a result of these changes, isolated systolic hypertension (ISH), wide PP hypertension by definition (SBP ≥ 140 and DBP < 90 mmHg), becomes the majority subtype of hypertension after the sixth decade of life. Although young women exhibit a much lower prevalence of hypertension than men of the same age, especially during the age of childbearing, women begin to narrow the hypertensive gap with men by their fourth decade of life even before the onset of the menopause, and surpass men by the seventh decade, so that almost 60% of all hypertensive individuals are women (Citation16).
Table II. Patterns of age-related changes in blood pressure.
How can pulse pressure help distinguish physiologic from pathologic vascular ageing?
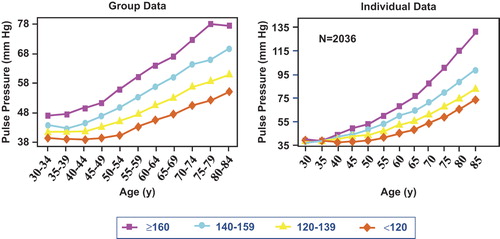
Just as there is a graded, continuous level of BP rather than a threshold that defines the onset of CVD risk, the same continuous relationship is true for physiologic and pathologic arterial ageing. The Framingham findings support the concept of an interaction between ageing and established hypertension in the progressive fall in DBP and continued rise in SBP after age 50–60 years (Citation16). Subjects with mean baseline BPs of 111/70 mmHg at age 32 years (, group 1) had no rise in PP and only minimal rise in MAP from age 30–49 years. Nevertheless, this group of subject with optimal BP at baseline showed a significant rise in PP and fall in DBP after the age of 60 years, presumably caused by an increase in large artery stiffness. In this example, mean SBP rose from 111 to 125 mmHg with an increase in PP of 14 mmHg over an interval of 50 years; this represents physiologic ageing. In contrast, subjects with mean baseline BPs of 130/84 mmHg (high-normal BP or prehypertension) at age 32 years (, group 4) showed a steeper rise in PP and a steeper fall in DBP after age 60 than was observed in group 1 subjects. In this second example, mean SBP rose from 130 to 173 mmHg with an increase in PP of 34 mmHg over the same interval of 50 years; this by contrast represents pathologic ageing. The divergent rather than parallel tracking pattern observed in all four SBP groups has been referred to as the “horse-racing effect”, there being a close correlation between the speed of the horse and its position in the race. These findings suggest a linkage between hypertension left untreated and the subsequent acceleration of large artery stiffness and pathologic ageing – thus creating a vicious cycle. There was as much as a 15–17-year acceleration of ageing based on the rates of change in PP when comparing group 4 with group 1 subjects (). The most important clinical implications that can be derived from this study are that after the sixth decade of life, (i) increasing PP and decreasing DBP are surrogate measurements for large artery stiffness: physiologic ageing occurred in group 1 subjects and accelerated pathologic ageing was present in group 4 subjects; (ii) large artery stiffness rather than vascular resistance becomes the dominant hemodynamic factor in both normotensive and hypertensive subjects from age 50 onward.
Figure 1. Pulse pressure by age. Group averaged data in left panel and averaged individual regression analysis in right panel for all subjects and with deaths, myocardial infarction and congestive heart failure excluded. Curves plotted based on blood pressure predicted values at 5-year age intervals by systolic blood pressure groupings. Adapted from Franklin SS, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–315, with permission (Citation16).
Furthermore, the CARDIA study (Coronary Artery Risk Development in Young Adults) (Citation17) showed that individuals with high-normal BP (BP 130–139, DBP 85–89 mmHg) prior to age 35, in comparison with normotensive individuals matched for age, developed a significantly higher coronary calcium score 20 years later as a sign of coronary atherosclerosis; this relationship was independent of other coronary heart disease (CHD) risk factors, and consistent across race and sex subgroups. This suggests that young adulthood is a critical period where even small elevation in BP can result in increased arterial stiffness and pathological arterial ageing, with or without associated CVD events, by the time one reaches middle age.
The use of PWV as the gold-standard for assessing arterial stiffness
Aortic PWV is a well-defined method for measuring arterial stiffness that can be determined from measurements of pulse transit time and the distance traveled by the pulse between the common carotid and femoral arteries (Citation18). Indeed, aortic PWV (carotid-to-femoral; c-f) can be measured by applanation tonometry, mechanotransducer or by Doppler probes, and is regarded as the gold standard for determining arterial stiffness that is independent of wave activity. The method is simple to use, does not require a skilled technician, is relatively inexpensive, is gender-independent, and is very reproducible but requires a stable cardiac rhythm for appropriate recording. Aortic PWV increases exponentially with ageing, and therefore, is a sensitive indicator of physiologic stiffness after the age of 50–60 years (Citation19). Unlike c-f PWV, carotid-to-brachial PWV does not increase with ageing, demonstrating that the upper extremity, primarily containing muscular arteries, does not show age-related changes in comparison with the highly elastic containing thoracic aorta and its branches (Citation19). Aortic PWV must be corrected for MAP, and there may be measurement problems in the presence of obesity, peripheral artery disease and cardiac arrhythmias, for example atrial fibrillation.
During the past several years, there have been published a number of longitudinal studies showing that aortic PWV has independent predictive value for CVD events, including persons with end stage renal disease (Citation20) and essential hypertension (Citation21). Benetos et al. (Citation22), using c-f PWV measurements of aortic stiffness in a 6-year longitudinal study, found that normotensive subjects < 50 years of age showed no evidence of increasing aortic stiffness, whereas the presence of high BP in the same young age group was associated with accelerated progression of aortic stiffness. Furthermore, the annual rates of progression in PWV were higher in treated hypertensives than in normotensive subjects. In addition, these investigators showed that MAP, a surrogate measure of PVR, did not increase throughout the 6-year follow-up, but PWV progression was more than three times greater in the poorly controlled compared with the well-controlled hypertensive subjects. Therefore, the Framingham Heart Study (Citation16) and Benetos et al. (Citation22) studies, independently, suggest a linkage between incompletely treated or untreated hypertension and the subsequent acceleration of age-related large artery stiffness – a measure of accelerated, pathological arterial ageing. Importantly, the increase in c-f PWV, a longitudinal measurement of the rate of arterial stiffening and a biomarker of pathologic ageing, reflects not only the elevation in BP, but also other CVD risk factors that cluster with the rise in BP. According to recent findings in meta-analyses, increased c-f PWV is an independent risk marker of CVD (Citation23), and the risk threshold should be 10 m/s, not 12 m/s as previously suggested (Citation18), based on recent consensus (Citation24). Reference values for European populations, stratified for age groups and sex, were published in 2010 by a collaborative group, based on data from 1400 healthy, normotensive subjects (Citation25).
Brachial pulse pressure as an independent predictor of CVD events
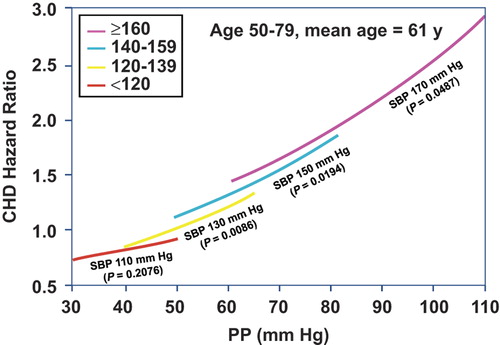
Using almost the same Framingham cohort as in the previous study, 1924 men and women between 50 and 79 years of age at baseline with no clinical evidence of CHD and free from antihypertensive drug therapy, were followed for up to 20 years () (Citation26). In this population, CHD risk was inversely correlated with DBP at any level of SBP > 120 mmHg, suggesting that PP was an important component of risk. The Framingham study supports the findings of earlier workers (Citation27–29) that PP may be useful as an adjunct to SBP in predicting risk and that CHD events are more related to the pulsatile stress of elastic artery stiffness during systole (as reflected in a rise in PP) than the steady-state stress of resistance during diastole (as reflected in a parallel rise in SBP and DBP). Seven additional publications, including a total of 12 different databases from around the world, have clearly shown an inverse relation with DBP so that PP becomes superior to the reference SBP in predicting total and cardiovascular mortality (Citation30–36), including a meta-analysis of eight trials by Staessen et al. (Citation36). Furthermore, the value of PP in predicting risk in the elderly has been confirmed by 24-h conventional (Citation36), intra-arterial (Citation35) and ambulatory BP monitoring (Citation37). Indeed, there are now multiple studies showing that PP is an independent predictor of cardiac complications (i.e. left ventricular hypertrophy, atrial fibrillation, systolic and diastolic dysfunction, and heart failure); large artery complications (myocardial infarctions, stroke); and microvascular complications (white matter lesions, cognitive impairment, dementia and chronic kidney disease).
Figure 2. Joint Influences of systolic blood pressure (SBP) and diastolic blood pressure (DBP) risk. Coronary heart disease (CHD) hazards ratios were determined from the level of DBP within SBP groups. Hazard ratios were set to a reference value of 1.0 for SBP of 130 mmHg and DBP of 80 mmHg and plotted for SBP values of 110, 130, 150, 170 mmHg, respectively. The p-values were for the β coefficients for model. All estimates were adjusted for age, sex, body mass index, cigarettes smoked per day, glucose intolerance and total cholesterol/high density lipoprotein. Adapted with permission from Franklin SS, et al. Circulation 1999;100:354–360 (Citation26).
Single vs combined blood pressure components and risk for pathological vascular ageing
The Framingham Heart Study examined the CVD predictive value of combined BP components vs single ones in a 2009 publication (Citation38) that was designed to maximize statistical power and provide more semiquantitative information on pathological vascular ageing. First, the study population was significantly larger: it consisted of the original (n = 4700) and offspring (n = 4897) cohorts, free of CVD events and without antihypertensive therapy over a 50-year period. Second, instead of only addressing CHD events, endpoints were enlarged to include a variety of cardiovascular events. In total, there were 1439 CVD events that consisted of myocardial infarction, thrombotic and hemorrhagic stroke, heart failure, CHD and CVD deaths. Third, pooled logistic regression analysis was used within 12 serial 4-year intervals from 1952 to 2000, using a new index examination for determining baseline BP for each 4-year cycle; this approach maximized person- observations (41,525 multiple person-observations). Fourth, models based on JNC-6 categories for SBP and various DBP categories, utilizing all 41,524 person-observations and 1439 CVD events, were constructed with optimal BP (SBP < 120 and DBP < 80 mmHg) serving as the reference group in order to explore the semiquantitatve value of high SBP and low DBP values on model prediction of pathological vascular aging (Citation38).
Categorical models in 6 × 6 cross-classification bar graphs to test for odds for the likelihood of CVD were constructed for SBP and DBP () and for PP and MAP (), adjusted for age, sex, total cholesterol, smoking, body mass index, diabetes and secular trend. Using the combination of BP components in and , respectively, rather than single BP components separately, improved the fit for predicting CVD risk. Introducing the interaction terms in and , respectively, further improved the fit over the main effects of the two-component models, indicating that the effect of one BP component on risk varied accordingly to the level of the other. There were no significant age or sex interactions with secondary analysis in modeling CVD risk, presumably because of maximum 4-year durations of each cycle. Neither models outperformed the other, which was not unexpected, since PP and MAP are derived from measured SBP and DBP.
Figure 3. Odds ratios for the likelihood of a cardiovascular event with combined systolic blood pressure (SBP) and diastolic blood pressure (DBP) categories in a 6 × 6 cross-classification bar graph, adjusted for age, sex, total cholesterol, smoking, body mass index, diabetes, and secular trend. An interaction term of SBP× DBP improved the model fit. From Franklin SS, et al. Circulation. 2009;119:243–250, with permission (Citation38).
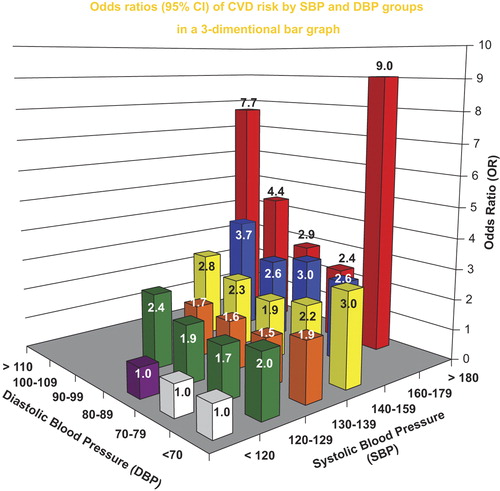
Figure 4. Odds ratios for the likelihood of a cardiovascular event with combined pulse pressure (PP) and mean arterial pressure (MAP) categories in a 6 × 6 cross-classification bar graph, adjusted for age, sex, total cholesterol, smoking, body mass index, diabetes and secular trend. An interaction term PP × MAP improved the model fit. From Franklin SS, et al. Circulation. 2009;119:243–250, with permission (Citation38).
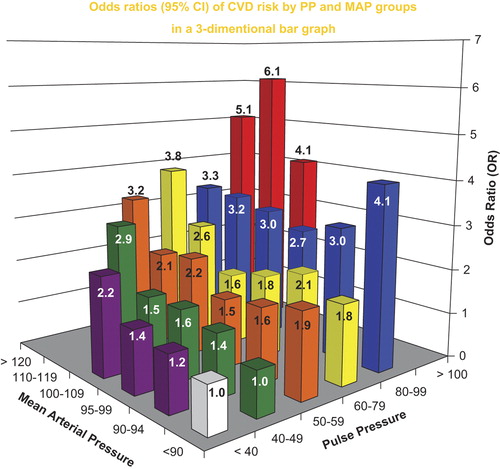
In the SBP+ DBP model, CVD risk increased at both the low and high extremes of DBP when combined with increased SBP in the two-component model. Therefore, there is a DBP J-curve for CVD risk and pathological ageing that is independent of antihypertensive therapy and antecedent CVD events. The J-curve relation to CVD risk that is associated with DBP presumably reflects increased arterial stiffness as manifested by a low DBP and by definition, a wide PP. It was concluded that both two-component models were superior to any single BP component in predicting CVD risk because they assessed both stiffness and resistance (afterload). A single BP component cannot do this, although SBP is the single best. Not surprisingly, single BP components as predictors of CVD risk in prior studies examined a limited spectrum of the overall hypertensive population by age, sex and other covariates. When PP, a measure of stiffness, was combined with MAP, a measurement of vascular resistance, one could relate the two major physiologic components of hydraulic load to clinical outcome as measurements of pathological ageing. Furthermore, this approach allowed for the semiquantitative assessment of accelerated pathological vascular ageing associated with either a very low or very high DBP: the former a measure of very great arterial stiffness and the latter a measure of very high PVR.
In the models based on JNC-6 categories for SBP and DBP groupings (), SBP is usually superior to DBP as a predictor of CVD risk; however, very low or very high DBP add to the SBP risk. Indeed, a DBP < 70 mmHg can add approximately 20 mmHg of SBP risk, i.e. a potential shift from pre-hypertension to stage 1 hypertension or from stage 1 to stage 2 hypertension. By the same token, a DBP > 100 mmHg vs 90–99 mmHg added considerable increased CVD risk secondary to increased resistance, despite somewhat lower SBP values ().
Table III. Prediction of cardiovascular disease (CVD) events by European Society of Hypertension (ESH) and Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure-6 (JNC-6) stages.
The importance of PP as a risk predictor was not emphasized in the 2003 JNC-7 recommendations (Citation39). The Framingham 2009 findings support the 2007 European Guidelines for the Management of Arterial Hypertension (Citation40) as a high-risk designation when elevated SBP is associated with DBP of < 60 to 70 mmHg and further suggest that high- risk may also apply to those individuals with pre-hypertension and DBP < 70 mmHg. These findings may represent important insights for improved preventative and therapeutic care that may minimize pathological vascular ageing.
The role of central blood pressure
There are pitfalls in the use of peripheral brachial BP components, especially SBP and PP, in predicting CVD risk. First, increased PP and the development of ISH are late manifestations of CVD risk and of pathologic arterial ageing, at a time when therapeutic intervention may have only suboptimal benefit. Second, the alerting white-coat reaction tends to falsely inflate PP due to a greater rise in SBP than DBP, especially when BP is measured by a physician or a nurse (Citation37). Third, and most important, pressure amplification, secondary to the summation of incident and reflected waves, may be a significant hindrance to the equating of peripheral to central SBP and PP.
The technique of applanation tonometry to measure central pressures, together with PWV, has been incorporated in the SphygmoCor system (AtCor Medical, Sydney, Australia) (Citation18). This widely used system can measure direct carotid or radial artery wave forms; the latter are technically easier to measure and with a validated generalized transfer function, central pressures can be calculated from the peripheral values (Citation41). Because MAP is almost identical in peripheral and central arteries, values computed from the applanated carotid artery waveforms can be used to calibrate MAP at the brachial artery; this permits central BP to be calculated more accurately (Citation42). As an example of the predictive value of applanation tonometry, central PP and pressure amplification were superior to brachial PP in predicting survival in end-stage renal disease patients maintained on hemodialysis therapy (Citation43), and in essential hypertension (Citation44,Citation45). Indeed, a study of variability between central (aortic) and peripheral (brachial) BP has shown that > 70% of persons with high-normal brachial BP had similar aortic pressures as those with stage 1 hypertension (Citation46). Furthermore, central BP may be a more important target than brachial BP for assessing the efficacy of antihypertensive therapy and this could have potential therapeutic importance in determining optimal BP control, especially in the young. However, the amplification phenomenon is maximal in young adults and may become minimal by middle age, especially with pathological ageing and increased arterial stiffness. Indeed, brachial and central PPs were almost identical in the observational Framingham population (Citation47).
These various measures of arterial stiffness should not be used interchangeably, because they do not necessarily measure the same modality in different pathological states. In general, there is some advantage in obtaining aortic PWV and central BPs in the same individual in order to best characterize the separate components of arterial stiffness and wave reflection. However, many additional studies will be necessary to determine the sensitivity, selectivity and cost of central BP and c-f PWV vs conventional brachial BP measurements before recommending that these new technologies are ready for use by primary care physicians; for now, these techniques are useful research tools.
Distribution of hypertension by age, sex and subtype in the US population
Approximately 65 million individuals in the USA and 1 billion worldwide are affected by hypertension (Citation48). Due to ageing of populations and the advent of effective antihypertensive therapy, there has been a shift toward a more slowly evolving form of hypertension that is predominately systolic in nature and affects middle-aged and older persons. The National Health and Nutrition Examination Survey (NHANES III, 1988–91) (Citation49) showed that three out of four adults with hypertension were age 50 or older. Moreover, about 80% of untreated or inadequately treated individuals with hypertension from age 50 onward had ISH, which by definition represents wide PP hypertension (Citation49). Of particular interest is the transition of hypertension subtype with increasing age. From NHANES IV data (), the most predominant form of hypertension among those age < 50 years are isolated diastolic hypertension (IDH) (SBP < 140 mmHg and DBP ≥ 90 mmHg) and systolic–diastolic hypertension (SDH) (SBP ≥ 140 mmHg and DBP ≥ 90 mmHg), which together account for approximately 80% of persons with hypertension from age 18 to 49 years (Citation50). Beginning at age 50, the most predominant form of hypertension is ISH, accounting for more than three-fourths of those with hypertension aged 50–59, approximately 80% of hypertension in those aged 60–69, and approximately 90% of those with hypertension aged ≥ 70 years. Thus, ISH is the most common subtype of hypertension. Furthermore, a Framingham study showed that normotensive persons reaching age 65 had a 90% lifetime risk of developing hypertension, almost exclusively of the ISH subtype, if they lived another 20–25 years (Citation50).
Figure 5. Frequency distribution of untreated hypertension individuals by age and hypertension subtype. Numbers at the top of bars represent the overall percentage distribution of all subtypes of untreated hypertension in the age group (NHANES III, 1988-1994). Blue bars: IDH, isolated diastolic hypertension (SBP < 140 and DBP ≥ 90 mm Hg); Red bars: SDH, systolic-diastolic hypertension (SBP ≥ 140 and DBP ≥ 90 mm Hg); Yellow bars: isolated systolic hypertension (SBP ≥ 140 and DBP < 90 mm Hg). From Franklin SS, et al. Hypertension 2001;37:869-874, with permission (Citation49).
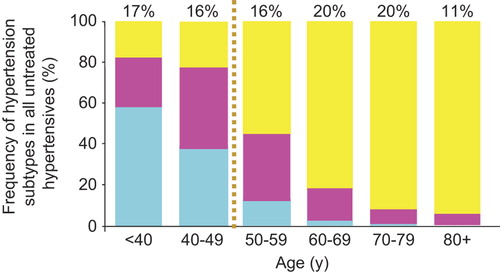
Therefore, most hypertensive patients fall into one of two categories: first, a smaller (26%), younger (age < 50 years), predominantly male (63%) with diastolic hypertension out of proportion to systolic hypertension (primarily IDH and SDH); second, a larger (74%), older (age ≥ 50 years), predominantly female (58%) with systolic hypertension out of proportion to diastolic hypertension (primarily ISH) (Citation48).
Two pathways for the development of ISH
The NHANES III survey (Citation49) showed that ISH becomes the dominant hypertensive subtype by the decade of age 50–59 years. As suggested by their age-dependent divergent patterns of onset, essential hypertension and ISH may be two distinct disorders with significant overlap. People who have had untreated or poorly treated diastolic hypertension at a younger age may develop ISH as they become older and their arteries become stiffer, but data from the Framingham Study suggests that only about 40% of patients with “high peripheral resistance” diastolic hypertension convert to ISH in this manner (Citation51). The majority of people who developed ISH never go through a stage of diastolic hypertension. In summary, diastolic hypertension (essential hypertension) and ISH are two distinct disorders with significant overlap. Both conditions are associated with accelerated pathologic vascular ageing.
Can accelerated vascular stiffening predict incident hypertension?
Using PWV to measure baseline arterial stiffness, Najjar et al. (Citation52), followed 449 normotensive or untreated hypertensives (mean age 53 years) for an average follow-up of 4.9 years. After adjusting for covariates of age, body mass index and MAP, PWV was an independent determinant of the longitudinal increase in SBP and of incident hypertension (hazard ratio, HR = 1.10 per 1 m/s increase in PWV, 95% confidence interval 1.00–1.30, p = 0.03). Not only can hypertension cause accelerated pathologic arterial ageing, but pathologic ageing of the vascular tree can occur prior to a rise in BP from normotensive to hypertensive levels (as a cause of incident hypertension), as shown in a recent longitudinal follow-up study from Framingham (Citation53). These findings suggest that cross-sectional or perhaps longitudinal assessment of PWV, in conjunction with calculation of global CVD risk scores, may identify normotensive individuals who should be targeted for intervention aimed at preventing or delaying the progression of subclinical arterial stiffening, pathologic ageing, and the onset of hypertension, for example members of risk families for CVD.
Epidemiological findings from Sweden
In Sweden, the existence of 10-digit personal identification numbers has made it possible to conduct and follow large population-based cohort studies over the last few decades. From a population-based study in the Värmland county, Sweden, between 1962 and 1965, in all 18,429 men and 19,414 women at the age of 50 or older were selected and followed for first CVD event until 2005 (Citation54). The biological interactions between sialic acid (SA), a marker of general inflammation, and PP were investigated. Adjustments were made for conventional risk factors, MAP and socio-economic status. The mean age (± standard deviation, SD) was 59.5 ± 6.5 years and the number of incident CVD event in men and women were 3641 and 3227, respectively. No biological interaction was seen between PP and SA. In men, the adjusted HR for PP was 0.92 (95% CI 0.88–0.96), p < 0.0001 for one SD of PP, and 1.09 (95% CI 1.05–1.13, p < 0.0001) for one SD of SA. In women, the corresponding figures were 1.02 (95% CI 0.97–1.07, p = 0.48) and 1.09 (95% CI 1.05–1.13, p < 0.0001). The risk induced by PP was highly affected by MAP. This suggests that both estimated arterial stiffness and inflammation contribute through different pathways to risk of CVD.
Smoking increased the risk associated with elevated SBP in another population-based study from southern Sweden, showing the interaction between risk factors (Citation55). In the same cohort, based on a study of predictors for increase of elevated PP over a 25-year period in a population-based study it was revealed that hyperglycemia was a significant long-term predictor (Citation56).
Value of lifestyle intervention
A variety of lifestyle interventions have been shown to lower BP and attenuate pathologic arterial ageing, with the most effective being successful weight reduction in overweight and obese hypertensives (Citation57). Unfortunately, most patients are refractory to successful weight reduction and even when partially successful tend to have a high percentage of recidivism within a year of losing weight. The older hypertensive patients are usually more salt sensitive than the young, especially in persons with ISH. Except for the unusual patient, lifestyle intervention is generally unsuccessful in correcting ISH fully. However, in addition to partial reduction in BP, lifestyle intervention may reduce the need for extensive antihypertensive therapy and minimize associated cardiovascular risk factors. It should be emphasized that lifestyle intervention is more likely to be successful in preventing rather than in reversing hypertension when initiated early at the high-normal BP level.
Therapeutic intervention in elderly persons with ISH
During the past few decades, the therapeutic approach in elderly hypertensive patients has changed markedly. In the early 1970s, prevailing wisdom questioned the benefit of antihypertensive agents in patients over 65 years of age with ISH. Beginning in the early 1990s, the publication of three major studies that specifically addressed the treatment of ISH in older patients changed the perception of the significance of SBP control and how it might attenuate pathologic arterial aging. In 1991, the landmark Systolic Hypertension in the Elderly Program (SHEP) double-blind, placebo-controlled study first established that older patients with ISH benefited from treatment (Citation58). The Syst-Eur (Citation59) and the Syst-China (Citation60) trials, also double-blind and placebo-controlled studies, corroborated these findings. Staessen and colleagues (Citation61) conducted a meta-analysis of the 11,825 patients aged 60 years or older who participated in these three major trials. This analysis found that antihypertensive treatment significantly reduced fatal and non-fatal coronary events by 25%, fatal and non-fatal strokes by 37%, all cardiovascular events by 32%, cardiovascular mortality by 25% and total mortality by 17% (Citation60). Additionally, a highly significant 49% reduction in fatal and non-fatal heart failure was reported from the SHEP study (Citation58). These studies negate prior assumptions that age- related changes in SBP are benign and reinforce the emerging paradigm that treatment will benefit patients with elevated SBP, even when they have normal or low DBP. Furthermore, the benefit to risk ratio of antihypertensive therapy is higher in the elderly than in younger or middle-aged patients.
Currently, life expectancy in the USA is 77 years, raising the question of the benefit of antihypertensive agents in patients over this age. A meta-analysis of six major trials that included 1670 patients of age 80 years or older suggested that even the very old may benefit from antihypertensive treatment (Citation62). In these patients, active treatment produced a 34% reduction in stroke (p = 0.014), a 39% reduction in heart failure (p = 0.01), and a 22% reduction in major cardiovascular events (p = 0.01). The reduction in coronary events was not statistically significant and a non-significant 6% increase in mortality was observed (Citation62). Evidence from this meta-analysis was strong for older patients with SBP of at least 160 mmHg (stage 2 ISH).
The HYVET study (Citation63), a more definitive intervention trial of the very old (from age 80 to 105) utilized a randomized, double-blind, placebo- controlled protocol in 2100 rather healthy subjects, largely with ISH. This study showed clearly that stage 2 ISH should be treated. There was a significant reduction in both fatal and non-fatal strokes (− 34%), heart failure (− 72%), and reduction in both CV (− 27%) and all-cause mortality (− 28%) (Citation58), providing arguments in favor of also treating the “healthy” very old person with hypertension.
Once considered an inconsequential part of the ageing process, the development of ISH represents a late manifestation of increased arterial stiffness and pathologic ageing in the middle-aged and elderly population. Its inherent increased risk for vascular events highlights the importance of its control. Therapeutic reduction in SBP in these very elderly patients reduced vascular stiffness, pathological aging, and CVD morbidity and mortality. Thus, pathologic arterial ageing can be attenuated with effective therapy, regardless of advanced age.
Pathologic aging of the cerebral vasculature – cognitive decline
The Baltimore Longitudinal Study on aging showed that elevated PP and PWV were related to cognitive impairment, based on decline in verbal and non-verbal memory test scores, in non-demented, middle-aged individuals (Citation64). These findings were confirmed in the Reykjavik Study, which showed that carotid PP, PWV and the pulsatility index were associated with lower memory scores (Citation65). Furthermore, a recent meta-analysis of three large intervention trials of antihypertensive therapy in elderly hypertensives (with and without prior strokes) shows that effective lowering of SBP significantly lowered the risk of vascular dementia and Alzheimer syndrome (odds ratio 0.75, 95% CI 0.64–0.94; p = 0.01) (Citation66). Thus, early treatment of hypertension by decreasing pathologic ageing of cerebral blood vessels may protect against both dementia and stroke, as well as cognitive decline according to a recent statement by the American Heart Association, AHA (Citation67).
Global CVD risk treatment to minimize pathologic vascular aging
There is a spectrum of CVD risk starting with early pathologic arterial ageing and stiffness prior to the development of high-normal BP or hypertension (primordial risk); high-normal BP or hypertension in the absence of target organ damage (subclinical disease); subclinical disease diagnosed by echocardiography (left ventricular hypertrophy), ultrasound (increased carotid intima-media thickness), urinalysis (proteinuria), ankle–brachial index (peripheral artery disease) and c-f PWV (accelerated arterial stiffness, EVA); and ultimately proceeding to CVD events (heart, kidneys, brain and vasculature). The therapeutic approach to this spectrum of progressive pathologic ageing of the CV system would start with lifestyle intervention and eventually add targeted drug therapy.
The Steno-2 study (Citation68) is an illustration of a successful global risk factor treatment approach in type 2 diabetes. Investigators randomly assigned 160 patients with type 2 diabetes and microalbuminuria to either standard or intense therapy for control of blood glucose, lipids and BP. At the conclusion of the 13-year study, there was a greater reduction in CVD deaths (HR = 0.43, 95% CI 0.19–0.94; p = 0.04) and CVD events (HR = 0.41, 95% CI 0.25–0.67; p < 0.001) in the intensively treated group compared with the standard treated group. This benefit was for both macro- and microvascular complications and the benefit from treating all three abnormities outweighed the benefit from treating any single abnormality. Since the arterial stiffening and pathologic aging in these diabetic patients was driven by abnormalities of BP, lipids and glycemia, optimal benefit could be obtained only by simultaneous effective treatment of all three modalities.
Conclusions
Early, accelerated and pathologic arterial ageing can occur prior to and in association with elevated BP and commonly clusters with other CVD risk factors. Increased PVR and arterial stiffness in large elastic arteries are two important hemodynamic abnormalities that accelerate pathologic ageing. The proportion of increased resistance vs increased stiffness varies with age, sex and a variety of covariates that include obesity, metabolic syndrome, diabetes and prior CVD events. Pathologic arterial ageing occurs predominantly with increased vascular resistance in the early adult years (more often in men) and predominantly with increased stiffness in the later adult years (more often in women). This leads to the cardiovascular ageing continuum (Citation69). Pathologic ageing over many years predisposes to later CVD events, including cogitative impairment. Early and aggressive treatment of persons at risk may provide greater protection against pathologic vascular ageing of heart, kidneys and brain, and hence delay or prevent later CVD events (Citation70). The establishment of the optimal level of BP to initiate antihypertensive therapy and optimal target goal of therapy to minimize pathologic arterial ageing are questions that will require further investigations and randomized studies (Citation71–73). The prevention of EVA will probably also require control of other risk factors, including hyperglycemia, to achieve maximum vascular protection. These studies are waiting to be planned and launched. In addition, a better understanding of the associations between EVA and genetic variation of arterial stiffness and the association with telomere attrition (Citation74,Citation75) could lead to new insights also in potential for prevention.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This review was supported by the Research Council of Sweden (grant 521-2010-2917) to PN.
References
- Snideman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1551.
- Nilsson PM. Early vascular aging (EVA): Consequences and prevention. Vasc Health Risk Manag. 2008;4:547–552.
- Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: The EVA syndrome (review). J. Hypertens. 2008;26:1049–1057.
- Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention (Brief Review). Hypertension. 2009;54:3–10.
- Fagard RH, Van Den Froeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–807.
- Kannel WB, Vasan R, Levy D. Is the relation of systolic blood pressure to risk of cardiovascular disease continuous and graded, or are there critical values?Hypertension. 2003; 42:453–456.
- Prospective studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- McMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease: Part 1. Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774.
- Levy BJ, Ambrosio G, Pries AR, Struijker-Boudier HAJ. Microcirculation in hypertension. A new target for treatment?Circulation. 2001;104:735–740.
- Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion. A pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976.
- Nichols WW, O’Rourke MF. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles. 5th ed. London: Hodder Arnold; 2005.
- Kannel WB. Risk stratification in hypertension: New insights from the Framingham Study. Am J Hypertens. 2000;13(1 Pt 2):3S–10S.
- Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter J, et al. Metabolic syndrome amplifies the age- associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395.
- Schillaci G, Pirro M, Faudo G, Mannarino MR, Savarese G, Pucci G, et al. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension. 2005;45:1078–1082.
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population: Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313.
- Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315.
- Pletcher MJ, Biffins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, et al. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149:91–99.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760.
- Blacher J. Guerin AP, Pannier, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Benetos A, Asamopouolos C, Bureau J-M, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327.
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al.; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid–femoral pulse wave velocity. J Hypertens. 2012;30: 445–448.
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values”. Eur Heart J. 2010;31:2338–2350.
- Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999; 100:354–360.
- Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: A cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400.
- Fang J. Madhavan S, Cohen H, Alderman MH. Measures of blood pressure and myocardial infarction in treated hypertensive patients. J Hypertens. 1995;13:413–419.
- Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetiere P, et al. Pulse pressure. A predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415.
- Lee MT, Rosner BA, Weiss CT. Relationship of blood pressure to cardiovascular death: The effect of pulse pressure in the elderly. Ann Epidemiol. 1999;9:101–107.
- Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension. Prognostic information provided by pulse pressure. Hypertension. 1999; 34:375–380.
- Glynn RG, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772.
- Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089.
- Benetos A, Zureik M, Morcet J, Thomas F, Bean K, Safar M, et al. A decrease in diastolic blood pressure combined with an increase in systolic blood pressure is associated with a higher cardiovascular mortality in men. J Am Coll Cardiol. 2000;35:673–680.
- Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001;104:783–789.
- Staessen JA, Gasowski, Wang JG, Lutgarde T, Hond ED, Boissel JP, et al. Risks of untreated and treated isolated systolic hypertension in the elderly meta-analysis of outcome trials. Lancet. 2000;104:865–872.
- Verdecchia P, Schillaci G, Borgione C, Ciucce A, Pede S, Porcellati C. Ambulatory pulse pressure: A potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988.
- Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, et al. Single versus combined blood pressure components and risk for cardiovascular disease. The Framingham Heart Study. Circulation. 2009;119:243–250.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) report. JAMA. 2003;289:2560–2572.
- The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 Guidelines for the management of arterial hypertension. Eur Heart J. 2007; 28:1462–1536.
- Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38: 932–937.
- Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: A validation repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963.
- Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39: 735–738.
- Williams, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. CAFÉ Investigators, CAFÉ Steering Committee and Writing Committee. Differential impact of blood pressure lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFÉ) Study. Circulation. 2006;113: 1212–1225.
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The Strong Heart Study. Hypertension. 2007;50:197–203.
- McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace, Rowe CV, Cockcroft JR, Wilkinson IB. Central pressure: Variability and impact of cardiovascular risk Factors. The Anglo-Cardiff Collborative Trial II. Hypertension. 2008; 51:1476–1482.
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation. 2010; 121:505–511.
- Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives. Hypertension. 2001; 37:869–874.
- Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men. JAMA. 2002;287:1003–1010.
- Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS, et al. Predictors of new-onset diastolic and systolic hypertension. The Framingham Heart Study. Circulation. 2005;111:1121–1127.
- Najjar S, Scuteri A, Shetty V, Wright BA, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383.
- Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881.
- Khalili P, Sundström J, Franklin SS, Jendle J, Lundin F, Jungner I, et al. Combined effects of brachial pulse pressure and sialic acid for risk of cardiovascular events during 40 years of follow-up in 37,843 individuals. J Hypertens. 2012;30:1718–1724.
- Khalili P, Nilsson PM, Nilsson JA, Berglund G. Smoking as a modifier of the systolic blood pressure-induced risk of cardiovascular events and mortality: A population-based prospective study of middle-aged men. J Hypertens. 2002;20:1759–1764.
- Mokhtari A, Bellinetto-Ford L, Melander O, Nilsson PM. Determinants of increasing pulse pressure during 23 years’ follow-up as a marker of arterial stiffness and vascular ageing. Blood Press. 2008;17:291–297.
- Cushman W, Dubbert P. Nonpharmacologic approaches to therapy of hypertension. Endocrine Pract. 1997;3:106–111
- SHEP cooperative Research Group: Prevention of stroke by antihypertensive drug in older persons with isolated systolic hypertension. JAMA. 1991;265:3255–3264.
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomized double-blind comparison of placebo and the active treatment for older patients with isolated systolic hypertension. Lancet. 1997;350:757–764.
- Wang J-G, Staessen JA, Gong L, Liu L. Chinese trial on isolated systolic hypertension in the elderly. Arch Intern Med. 2000;160:211–220.
- Staessen JA, Wang JG, Thijs L, Fagard R. Overview of the outcome trials in older patients with isolated systolic hypertension. J Human Hypertens. 1999;13:859–863.
- Gueyffier F, Bulpitt C, Boissel J-P, Schron E, Ekbom T, Fagard R, et al. Antihypertensive drugs in very old people: A subgroup meta-analysis of randomized controlled trials. Lancet. 1999;353:793–796.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898.
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:991–994.
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility – Reykjavik study. Brain. 2011 Nov;134(Pt 11):3398–3407.
- Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400.
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al.; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591.
- O’Rourke MF, Safar ME, Dzau V. The cardiovascular continuum extended: Aging effects on the aorta and microvasculature. Vasc Med. 2010;15:461–468.
- Safar ME, Nilsson PM, Blacher J, Mimran A. Pulse pressure, arterial stiffness, and end-organ damage. Curr Hypertens Rep. 2012;14:339–344.
- Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, et al.; investigators. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: A meta-analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–1042.
- Boutouyrie P, Lacolley P, Briet M, Regnault V, Stanton A, Laurent S, et al. Pharmacological modulation of arterial stiffness. Drugs. 2011;71:1689–1701.
- Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: Needed clinical trials. Hypertension. 2012;60:518–522.
- Nilsson PM. Genetic and environmental determinants of early vascular ageing (EVA). Curr Vasc Pharmacol. 2012;10:700–701.
- Nilsson PM. Impact of vascular aging on cardiovascular disease: The role of telomere biology. J Hypertens. 2012;30 Suppl:S9–S12.
