Abstract
Background. HeartSCORE is a tool for assessing cardiovascular risk, basing its estimates on the relative weight of conventional cardiovascular risk factors. However, new markers of cardiovascular risk have been identified, such as aortic pulse wave velocity (PWV). The purpose of this study was to evaluate to what extent the incorporation of PWV in HeartSCORE increases its discriminative power of major cardiovascular events (MACE). Methods and results. This study is a sub-analysis of the EDIVA project, which is a prospective cohort, multicenter and observational study involving 2200 individuals of Portuguese nationality (1290 men and 910 women) aged between 18 and 91 years (mean 46.33 ± 13.76 years), with annual measurements of PWV (Complior). Only participants above 35 years old were included in the present re-analysis, resulting in a population of 1709 participants. All MACE – death, cerebrovascular accident, coronary accidents (coronary heart disease), peripheral arterial disease and renal failure – were recorded. During a mean follow-up period of 21.42 ± 10.76 months, there were 47 non-fatal MACE (2.1% of the sample). Cardiovascular risk was estimated in all patients based on the HeartSCORE risk factors. For the analysis, the refitted HeartSCORE and PWV were divided into three risk categories. The event-free survival at 2 years was 98.6%, 98.0% and 96.1%, respectively in the low-, intermediate- and high-risk categories of HeartSCORE (log-rank p < 0.001). The multi-adjusted hazard ratio (HR) per 1 − standard deviation (SD) of MACE was 1.86 (95% CI 1.37–2.53, p < 0.001) for PWV. The risk of MACE by tertiles of PWV and risk categories of the HeartSCORE increased linearly, and the risk was particularly more pronounced in the highest tertile of PWV for any category of the HeartSCORE, demonstrating an improvement in the prediction of cardiovascular risk. It was clearly depicted a high discriminative capacity of PWV even in groups of apparent intermediate cardiovascular risk. Measures of model fit, discrimination and calibration revealed an improvement in risk classification when PWV was added to the risk-factor model. The C statistics improved from 0.69 to 0.78 (adding PWV, p = 0.005). The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also determined, and indicated further evidence of improvements in discrimination of the outcome when including PWV in the risk-factor model (NRI = 0.265; IDI = 0.012). Conclusion. The results clearly illustrate the benefits of integrating PWV in the risk assessment strategies, as advocated by HeartSCORE, insofar as it contributes to a better discriminative capacity of global cardiovascular risk, particularly in individuals with low or moderate cardiovascular risk.
Introduction
Cardiovascular diseases are responsible for a high proportion of global morbidity and mortality, constituting a public health problem of first magnitude in the world (Citation1). In order to optimize preventive strategies at the population level, several organizations have developed risk scores to categorize patients into risk classes that will determine the therapeutic strategies to follow in order to reduce the risk of major cardiovascular events (MACE) (Citation2,Citation3). One of the most important tables for the current risk stratification, and reference table at the European level, is the Systemic COronary Risk Evaluation (HeartSCORE) (Citation2). This table of risk developed by the European Society of Cardiology estimates the risk of cardiovascular death at 10 years based on five traditional cardiovascular risk factors (age, gender, systolic blood pressure, cholesterol and smoking), supported on a risk function derived from 12 European cohort studies. However, the positive predictive value of this score has proved somewhat disappointing, particularly in individuals of apparent low cardiovascular risk (score < 5%). This limitation can be overcome with the inclusion of other risk markers that potentially may increase the discriminative capacity of SCORE and allow for the optimization of clinical decision making in primary prevention (Citation4). Given the recognition of this limitation, the guidelines for the prevention of cardiovascular disease from the European Society of Cardiology have advised the integration of other markers of subclinical target organ damage in clinical decision, allowing the risk categories obtained by SCORE to be adjusted to these new markers. Accordingly, this study aims to assess to what extent the integration of the aortic pulse wave velocity (PWV) in the HeartSCORE would increase its discriminative capacity for cardiovascular risk in a group of 2200 subjects enrolled in a population-based cohort study (Citation5). In fact, considering the arteries are the site, the target and the common denominator in cardiovascular disease, their evaluation becomes crucial in any strategy of defining the individual cardiovascular risk and clinical decision making (Citation6). This has assumed particular relevance in relation to the study of arterial stiffness, with a growing number of clinical studies pointing unequivocally to its connection with cardiovascular morbidity and mortality (Citation7–20). Despite the various parameters and indices of arterial stiffness currently available (Citation6), PWV has been accepted as the most relevant, bringing together a broad scientific support in combination with its unique methodological characteristics (simplicity, reproducibility and low cost). The relationship between PWV and cardiovascular risk is well documented in several clinical settings and in the general population (Citation7–20), being a strong and powerful predictor of cardiovascular events. To what extent its inclusion in routine cardiovascular risk screening adds discriminability to the conventional cardiovascular risk factors still needs further confirmation. The purpose of this study was to evaluate to what extent the incorporation of PWV over the HeartSCORE risk factors increases its discriminative power for MACE.
Methods
Population
This study represents a re-analysis of the EDIVA project database (Citation5), aiming to ascertain whether the inclusion of aortic PWV to the HeartSCORE adds discriminative capacity for MACE. The EDIVA project was established to raise awareness of PWV measurement, incorporating a training element and a monitoring structure and leading to the collection of data for statistical analysis (as described elsewhere (Citation5)). The EDIVA study, as a nuclear part of this broad project, was an epidemiological study assessing cardiovascular risk through sequential PWV measurement. The study population consisted of 2200 Portuguese nationals (1290 men and 910 women), aged between 18 and 91 years (mean 46.33 ± 13.76 years). For the present analysis, we selected from the original database, individuals aged above 35 years and without symptomatic cardiovascular disease, resulting in a cohort of 1709 individuals, 744 female and 944 males, mean age 51.68 ± 10.49 years (range: 35–85).
These patients were followed for about 22 months, with annual clinical evaluations.
The study's aims were explained to all participants and their informed consent was obtained. The methodology used to collect the data was approved by the Portuguese Data Protection Commission and the study was approved by the ethics committees of the hospitals involved.
Study design
The EDIVA study (Citation5) was a prospective, multicenter, observational study monitoring the occurrence of MACE – death, stroke, transient ischemic attack, myocardial infarction, unstable angina, peripheral arterial disease, revascularization or renal failure. Follow-up of the patients consisted of annual assessments including carotid–femoral PWV, blood pressure (BP), laboratory tests and clinical observation. At each consultation, the subjects’ weight and height were measured and body mass index (BMI) was calculated in kg/m2. BP was measured in a supine position and after a 10-min resting period, by an experienced operator and using a clinically validated (class A) sphygmomanometer (Colson MAM BP 3AA1-2®; Colson, Paris) (Citation21). The mean of three BP measurements was used in the analysis.
For each patient we calculated the 2-year risk of cardiovascular event using the components of the EuroSCORE risk factors (Citation2) refitted to the EDIVA study as described in the statistical analysis section. We then considered three risk categories: a SCORE < 1% corresponding to low risk, a SCORE ≥ 1 and < 5% to intermediate risk, and a SCORE ≥ 5% to high risk.
All participants underwent routine fasting laboratory tests. At the first consultation, they filled out a questionnaire concerning relevant personal and family history, smoking habits, alcohol consumption and medication.
Pulse wave velocity
Carotid–femoral PWV was determined using a Complior® device (Colson, Paris) in accordance with a previously described technique (Citation22). Briefly, PWV was based on the distance/time ratio (m/s) with the pulse wave measured simultaneously in the right carotid and right femoral arteries, the distance used being that between the sites where the pressure waves were recorded. Measurements were performed by the same operator and the quality of the recordings was evaluated by two independent observers with considerable experience of the method. The reproducibility of these estimates previously determined in our laboratory showed correlation coefficients better than 0.9 (0.98 and 0.95, respectively, for inter- and intra-observer differences) (Citation23).
Statistical analysis
The distribution of the variables was tested for normality using the Kolmogorov–Smirnov test, and for homogeneity of variance by Levene's test. Simple descriptive statistics were used to characterize the sample and the distribution of variables. Data are presented as mean ± standard deviation (SD), for continuous variables with normal distribution, median (interquartile range, IQR) for continuous variables without normal distribution, and frequency for categorical variables. Groups were compared using the χ2 test, for categorical variables, and the Student's t-test or the Mann–Whitney U test for quantitative variables, as appropriated.
Cox proportional hazards analysis was used to determine the association between PWV and time to first major cardiovascular event. Covariates were selected on the basis of components of the EuroSCORE (Citation2) and included the following at the baseline examination: age, sex, systolic BP, total cholesterol concentrations, smoking habits, presence of diabetes mellitus and use of antihypertensive therapy. All variables in the models were tested for linearity and proportional hazard assumption. Kaplan–Meier survival analysis was also applied with the cohort segregated according to tertiles of PWV and the refitted SCORE categories.
To compare the discriminatory power of PWV in addition to the SCORE risk model, we estimated measures of model fit, discrimination and calibration. Model fit was measured with the likelihood ratio test, the Akaike information criterion and the Schwartz's Bayesian information criterion; Harrell's C-index was used as a measure of discrimination. The Hosmer–Lemeshow test was used to check calibration of the models. In order to check the discrimination and reclassification improvement, we computed the predicted risk for all of the participants using a Cox model that included only the standard risk factors. Using predicted risk from this model, we defined cut points for risk groups based on the predicted risk in participants who experienced an event within 2 years. We cross-classified categories of risk on the basis of a model that included standard risk factors against those based on a model that added PWV. The net reclassification improvement (NRI) and the integrated discrimination index improvement (IDI) were then derived.
All analyses were conducted in Stata version 11. A value of p ≤ 0.05 was taken as the criterion of statistical significance for a 95% confidence interval.
Results
The cohort consisted of 1709 individuals, with a mean age of 51.68 ± 10.49 (ranging from 35 to 85 years), with a similar proportion of men and women. Baseline study cohort characteristics are shown in . About 58.6% of the cohort had arterial hypertension, 35.3% had dyslipidemia and 11.6 were diabetics. Smoking habits were seen in 14% of the study cohort individuals.
Table I. Baseline characteristics of the study cohort.
During a mean follow-up period of 21.42 ± 10.76 months, a total of 47 non-fatal MACE (2.1% of the sample) were recorded, including 25 cases of stroke, 18 of coronary events, two of renal failure and two of occlusive peripheral arterial disease. As expected, PWV was significantly higher in subjects with events than in those without them (11.82 ± 2.16 m/s vs 10.40 ± 2.01 m/s, respectively, p < 0.001). Patients with events also had higher BP levels, as predicted in . In a multivariable Cox regression analysis, adjusting the model for standard cardiovascular risk factors, PWV was an independent predictor of MACE, with a 86% increase in the risk of MACE per 1 − SD increase in PWV (hazard ratio, HR, per 1 − SD = 1.86; 95% CI 1.37–2.53, p < 0.001, cf. ). The incidence of cardiovascular events according to the refitted SCORE classification was 1.9% in the low-risk group, 2.6% in the intermediate-risk group and 5.5% in the high-risk group (p = 0.001).
Table II. Multiple-adjusted hazard ratios and 95% confidence intervals (CI) for cardiovascular risk factors increasing risk of major adverse cardiovascular events.
We then tested whether the addition of PWV would add discriminative capacity to the SCORE risk factors. The measures of model performance are presented in . Adding the aortic PWV to a baseline model including only age and sex produced a significant improvement in the discrimination of cardiovascular events, as depicted by the considered measures of model fit, discrimination and calibration. The C statistics improved from 0.69 to 0.78 (adding PWV, p = 0.005). Considering the impact of adding PWV to the risk-factor model, an overall discriminative improvement was also depicted. Model fit improvement was indicated by reductions in the log likelihood, the Akaike information criterion and the Schwartz's Bayesian information criterion. A discrimination improvement was also demonstrated, with an increase in the C statistics from 0.82 to 0.86 (p = 0.03), and calibration was also very good as shown by the Hosmer–Lemeshow test.
Table III. Measures of model fit, discrimination, and calibration for major adverse cardiovascular events adding carotid–femoral pulse wave velocity.
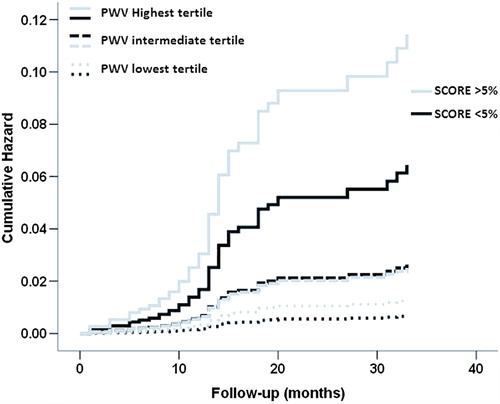
For further illustration of the reclassification improvement when adding PWV to the conventional SCORE risk-factors, we conducted a Cox regression analysis combining three SCORE classes (low, medium and high risk) with three tertiles of PWV (low, medium and high tertile). The cumulative risk for each of the considered categories was obtained, as depicted in , having the low SCORE and low PWV tertile as the reference category. For each of the considered SCORE categories, PWV strongly added discriminative capacity, allowing for a better classification of cardiovascular risk. This is particularly relevant when considering the low- and intermediate-risk categories, in which PWV could be crucial to optimize clinical decision making. This same aspect is well demonstrated in the Kaplan–Meier curves depicted in . When patients were grouped based on a 5% cut-off of the SCORE (< 5% low to intermediate risk; > 5% high risk) and tertiles of PWV, the probability of developing MACE increased with PWV tertiles in both SCORE categories. The category-free NRI was also determined, being 0.265 (p = 0.0001). Upward and downward reclassification with the addition of PWV to the standard model was seen respectively in 21.2% and 2.1% of participants with cardiovascular events, and in 7.8% and 13.4% of participants without cardiovascular events. The “clinical” NRI (over the intermediate-risk individuals) was 0.311, with 20% participants with events being upward reclassified with the inclusion of PWV. The IDI indicated further strong evidence of improvements in discrimination for the outcome when including PWV in the risk-factor model (IDI = 0.012, p = 0.002).
Figure 1. Cox regression coefficients of cumulative probability of a first major adverse cardiovascular event when participants were grouped according to tertiles of carotid–femoral pulse wave velocity and three SCORE risk classes.
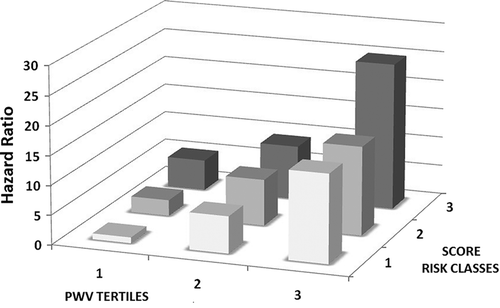
Figure 2. Kaplan–Meier plot of cumulative probability of major adverse cardiovascular events when participants were grouped according to SCORE categories (low- and intermediate-risk category – SCORE < 5%; high-risk category – SCORE > 5%) and stratified to tertiles of PWV (solid line – highest tertile; dashed lines – intermediate and lowest tertiles).
Discussion
Arterial distensibility is an important area in cardiovascular research, particularly because of its value for risk stratification in various clinical situations. Carotid–femoral PWV is without a doubt the best indicator of aortic stiffness (Citation6), and it is unequivocally related to cardiovascular mortality and morbidity in patients with diabetes, hypertension or renal failure, in the elderly, and in the general population (Citation7–20). Recently we published the results of the EDIVA cohort study (Citation5), confirming PWV as an independent marker of cardiovascular risk in the general population, with an adjusted HR of 1.316 per 1 m/s increase in PWV for non-fatal MACE. In the current re-analysis of the EDIVA database we wanted to stretch the implied relation of aortic PWV with cardiovascular risk, testing if its inclusion would add discriminatory information to the conventional risk factors integrated in the EuroSCORE charts.
Our data further demonstrates the importance of PWV for cardiovascular risk stratification in the general population, over and above conventional cardiovascular risk factors. In fact, adding PWV to the SCORE risk-factor model significantly enhanced the discrimination of the patients, particularly over the low and intermediate-risk categories. The measures of model fit and discrimination clearly depicted an improvement when PWV was added to the SCORE criteria, and model calibration was shown to be very good. Furthermore, the IDI also stressed the improvements in discrimination for the outcome when including PWV in the risk-factor model. Albeit these favorable results, we have to consider that the EDIVA outcome data specifically reflected short-term and non-fatal outcomes, whilst the EuroSCORE is based on the prediction of 10-year cardiovascular mortality. Even though the analysis refitted the EuroSCORE to the shorter follow-up, the EDIVA outcomes provide only non-fatal cardiovascular events, and the number of events was small conditioning the statistical power of the analysis. In that sense, the reported results give added confirmation to PWV as a powerful marker of sub-clinical organ lesion and a powerful independent predictor for non-fatal cardiovascular events. Meanwhile, other studies have also demonstrated a strong association of PWV and cardiovascular risk (Citation7–20). In fact, a recently published analysis of a cohort of 2232 participants in the Framingham Heart Study (Citation19), showed that the risk of cardiovascular events increases with increasing quartiles of PWV, so that patients at the highest quartile of PWV (PWV > 11.8 m/s) had a 48% increased risk of cardiovascular events (95% CI 1.16–1.91, p = 0.002), compared with the ones at the lowest quartile (PWV < 7.7 m/s). Additionally, and in line with our results, they showed that increased PWV provides incremental cardiovascular risk stratification beyond that provided by standard Framingham risk factors. Similar results were also reported by Sehestedt et al. (Citation24) in a cohort of 1968 followed for about 12 years, demonstrating that subclinical organ damage (including PWV) predicts cardiovascular death independently of the SCORE classification, and that the combination of both SCORE and subclinical organ damage markers improves cardiovascular risk prediction (Citation24). Moreover, two recent meta-analyses addressing the predictive capacity of PWV for MACE unequivocally expressed PWV as a strong and independent prognostic predictor in different clinical contexts (Citation20,Citation25).
Given the accumulated data, we can argue unequivocally in favor of integrating PWV in clinical practice as a key variable to manage cardiovascular risk in the overall population. In fact, considering the clinical profiles of the patients enrolled in the published papers so far, we can comfortably sustain that PWV is equally important in patients with manifest cardiovascular risk (diabetic, hypertensive, dyslipidemic, renal failure and obese patients) (Citation7–13) and in primary prevention of general population cohorts (Citation5,Citation14–18).
Albeit this impressive confirmation of PWV as a true cardiovascular risk factor, its use in clinical practice is still far from what would be expected considering the available body of evidence. Two main factors could explain this apparent paradox, in our understanding. The first one is the limited availability of this technology, even though the cost/benefit relation is clearly balanced in favor of its use. The other factor derives from the absence of standard reference values and clinical recommendations regarding the therapeutic management of PWV. In a recent contribution of our centre (Citation5), we presented a table of reference values for PWV, for the Portuguese population, adjusted for age and gender, and considering a statistical definition rather that an operational one. The classification of PWV according to the proposed reference values was independently associated with MACE, after adjustment to standard cardiovascular risk factors. Boutouyrie et al. also proposed reference values to PWV, obtained from a sample of 16867 subjects, enrolled in 13 centers spread through eight European countries (Citation26). However, if we already have promising data regarding the establishment of reference values to be used in clinical practice (Citation26,Citation27), the extent as to how PWV could be a direct therapeutic target is still unknown and a crucial premise to be fulfilled (Citation28,Citation29).
In conclusion, PWV gives added value to the SCORE cardiovascular risk stratification, determining cardiovascular risk over and above the standard risk factors included in the SCORE algorithm. Considering the potential benefit of this better risk stratification for optimizing the therapeutic management of patients, PWV should be included as a routine evaluation in clinical practice. However, a better methodological standardization, the compliance to the available guidelines for arterial stiffness assessment, and the standardization of reference values, are aspects that need further consideration and refinement.
Acknowledgments
We are grateful to this company for the integrity and commitment they have shown in an exemplary collaboration between the pharmaceutical industry and postgraduate medical training. The authors sincerely thank Dr Cristina Matos Serra for her linguistic assistance.
Disclosures: The authors have no conflicts of interest to disclose.
The EDIVA Project was supported and sponsored by Clínica da Aveleira and Medinfar Farmacêutica, SA.
References
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Life. Geneva: WHO; 2002.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–3421.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Maldonado J, Pereira T, Polónia J, Silva JA, Morais J, Marques M. Arterial stiffness predicts cardiovascular outcome in a low-to-moderate cardiovascular risk population: The EDIVA (Estudo de DIstensibilidade VAscular) project. J Hypertens. 2011;29:669–675.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al., on behalf of the European Network for Non-Invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–2605.
- Blacher J, Guérin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999:99:2434–2439.
- Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050.
- Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol. 2001;12:2117–2124.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2002;37:1236–1241.
- Boutourye P, Tropeano AI, Asmar R, Gautir I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15.
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function?Circulation. 2002; 106:2085–2090.
- Laurent S, Katsahian S, Fassot C, Tropeano AL, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34: 1203–1206.
- Sutton-Tyrrel K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390.
- Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, et al. Pulse wave velocity predicts cardiovascular mortality: Findings from the Hawaii–Los Angeles–Hiroshima study. Circ. J. 2005;69:259–264.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670.
- Mattase-Raso FU, Van der Cammen TJ, Hofman A, Van Popele NM, Bos ML, Shalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006;113:657–663.
- Aatola H, Hutri-Kähönen N, Juonala M, Viikari J, Hulkkonen J, Laitinen T, et al. Lifetime risk factors and arterial pulse wave velocity: The Cardiovascular Risk in Young Finns Study. Hypertension. 2010;55:806–811.
- Mitchell G, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation. 2010; 121:505–511.
- Vlachopoulos C, Aznaouridis K, Stefanadis C.Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327.
- Pereira T, Maldonado J. Performance of the Colson MAM BP 3AA1-2 automatic blood pressure monitor according to the European Society of Hypertension validation protocol. Rev Port Cardiol. 2005;24:1341–1351.
- Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26: 485–490.
- Pereira T, Maldonado J. Comparative reproducibility of two generations of the Complior® device estimating carotid– femoral pulse wave velocity. Blood Press Monit. 2011;15: 316–321.
- Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Peterson C, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–891.
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson S, Boutouyrie P, et al. Prognostic value of carotid–femoral pulse wave velocity for cardiovascular events: An IPD meta-analysis of prospective observational data from 14 studies including 16,358 subjects. Artery Res. 2011;5:138–139.
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur Heart J. 2010;31: 2338–2350.
- Pereira T, Maldonado J, Polónia J, Alberto Silva J, Morais J, Marques M. A statistical definition of aortic pulse wave velocity normality in a Portuguese population: A subanalysis of the EDIVA project. Rev Port Cardiol. 2011;30:691–698.
- Gómez-Marcos MA, González-Elena LJ, Recio-Rodríguez JI, Rodríguez-Sánchez E, Magallón-Botaya R, Muñoz-Moreno MF, et al. Cardiovascular risk assessment in hypertensive patients with tests recommended by the European Guidelines on Hypertension. Eur J Prev Cardiol. 2012;19:515–522.
- Kesse-Guyot E, Vergnaud AC, Fezeu L, Zureik M, Blacher J, Péneau S, et al. Associations between dietary patterns and arterial stiffness, carotid artery intima-media thickness and atherosclerosis. Eur J Prev Cardiol. 2010;17: 718–724.
