Abstract
Background. The high-sensitivity cardiac troponin T (hs-cTnT) assay provides important prognostic information on cardiovascular diseases. Although hs-cTnT is associated with left ventricle (LV) hypertrophy (LVH), it has not been investigated in different LV geometric patterns incorporating normal LV structure and concentric remodeling in addition to LVH. Objectives. We aimed to investigate the possible association between hs-cTnT and LV geometric patterns in newly diagnosed hypertensive patients. Methods. We studied 306 patients with newly diagnosed hypertension (HT; mean age 51.7 ± 5.6 years) and 44 healthy control subjects (mean age 51.3 ± 4.7 years). Echocardiographic examination was performed in all subjects. Four different geometric patterns were determined in hypertensive patients according to LV mass index (LVMI) and relative wall thickness (RWth). hs-cTnT and other biochemical markers were measured in all participants. Results. The highest hs-cTnT values were observed in the concentric hypertrophy group compared with the control, normal geometry, concentric remodeling and eccentric hypertrophy groups (p < 0.05, for all). Also, hs-cTnT values of the eccentric hypertrophy group were higher than the control, normal geometry and concentric remodeling groups (p < 0.05, for all). Multivariate regression analysis showed that hs-cTnT was independently associated with LV geometry (β = 0.326, p = 0.001) as well as LVMI (β = 0.228, p = 0.010) and creatinine level β = 0.132, p = 0.012). Conclusion. hs-cTnT level is related not only to LVH but also to LV geometry in hypertensive patients. hs-cTnT levels may mediate poorer LV geometric patterns in hypertensive patients.
Introduction
A new generation of highly sensitive cardiac troponin (hs-cTn) assays has recently been developed, which allows for the detection of even minor myocardial necrosis with high precision (Citation1). The most of the patients with hypertension (HT) have elevated cardiac troponin T concentrations that are undetectable with conventional assays even without myocardial necrosis (Citation2). The increased diagnostic and prognostic accuracy of the hs-cTnT assay versus the conventional cTnT assay has recently been reported in hypertensive patients (Citation3). Also, hs-cTnT levels are strongly associated with left ventricle hypertrophy (LVH) in general population, hypertensive patients and different patients groups (Citation3–5).
There are four different geometric patterns of left ventricle (LV) in HT, and these geometric patterns are different for prognosis and LV function (Citation6,Citation7). LV geometric patterns incorporate normal LV structure and concentric remodeling in addition to LVH (eccentric hypertrophy and concentric hypertrophy) (Citation7). Although hs-cTnT level is associated with LVH in hypertensive patients (Citation3), it has not been investigated in different LV geometric patterns. Therefore, we aimed to investigate the possible association between hs-cTnT concentrations and LV geometric patterns in newly diagnosed hypertensive patients.
Methods
Study populations
Between January 2012 and June 2013, a total of 416 consecutive patients who admitted our outpatient clinic and having newly diagnosed essential HT were evaluated for the study. Previously diagnosed hypertensive patients were not evaluated for this study. Secondary causes of HT were investigated in all patients. According to history, physical examination and electrocardiographic findings, patients who had suspicion of coronary artery disease underwent stress tests. Of the 423 patients, 56 were excluded due to diabetes mellitus, 16 patients were excluded due to chronic renal insufficiency, 24 patients were excluded due to ischemic heart disease, 14 patients were excluded due to severe valvular heart disease (nine patients had severe aortic stenosis, three patients had moderate mitral regurgitation and two patients had moderate aortic regurgitation) and seven patients exclude due to secondary HT. Therefore, measurements were obtained from 306 (mean age: 51.7 ± 5.4 years, male/female: 136/170) patients with newly diagnosed essential HT in this study and 44 healthy controls (mean age: 51.3 ± 4.7 years, male/female: 20/24).
Hypertensive patients had three clinic blood pressure measurements (≥ 140/90 mmHg) taken at 1-week intervals in the absence of any previous antihypertensive treatment to exclude the pharmacological effects on hemodynamics or ventricular hypertrophy and function. The control group was completely healthy and had multiple normal blood pressure measurements (< 140/90 mmHg) and were age- and gender-matched in relation to the hypertensive patients. Any abnormalities on physical examination or ECG of healthy volunteers were excluded from the study. The local ethics committee assessed and approved the study and written informed consent for participation in the study was obtained from all individuals.
Exclusion criteria were secondary or malignant HT, heart failure, positive history or clinical signs of ischemic heart disease, positive effort test, positive myocardial perfusion scintigraphy, cerebrovascular disease, moderate or severe valvular heart disease, atrial fibrillation, use of any antihypertensive medication, renal insufficiency (serum creatinine: ≥ 1.5 mg/dl in men and ≥ 1.4 mg/dl in women), major non-cardiovascular diseases and known diabetes or fasting glucose ≥ 126 mg/dl.
Blood samples
Blood samples were obtained in the morning after a 20-min rest following a fasting period of 12 h. Glucose, creatinine and lipid profiles for blood samples were analyzed for each patient. High- sensitivity C reactive protein (hs-CRP) was measured using BN2 model nephelometer. hs-cTnT was measured using a fourth-generation assay on an Elecsys 2010/Cobas e 411 instrument (Roche Diagnostics, Mannheim, Germany). The limit of blank (LoB) was 3 ng/l, and values under the limit were routinely classified as 2.99 ng/l. The upper reference limit (99th percentile) was 14 ng/l, and the lowest concentration with a coefficient of variance (CV) ≤ 10% was 13 ng/l (Citation8). hs-cTnT level was divided into two groups according to median level of hs-cTnT (higher hs-cTnT > 5.27 ng/l and lower hs-cTnT < 5.27 ng/l).
Echocardiography
Standard two-dimensional and Doppler echocardiographies were performed using a commercially available echocardiographic machine (Vivid 7R GE Medical System, Horten, Norway) with a 2.0–3.5-MHz transducer. Measurements were made during normal breathing at end expiration. LV end-systolic (LVSd) and end-diastolic diameters (LVEDD), end-diastolic interventricular septal thickness (IVSth), and end-diastolic LV posterior wall thickness (PWth) were measured at end-diastole according to established standards of the American Society of Echocardiography (Citation9). LV ejection fraction (EF) was determined by the biplane Simpson's method (Citation10). LV mass (LVM) was calculated using the Devereux formula (Citation11):
Then, the LV mass index (LVMI, g/m2) was obtained with the following formula: LVM/body surface area (BSA). LVH was defined according to more stringent criteria as LVMI values exceeding 125 g/m2 in men and 110 g/m2 in women (Citation12). Relative wall thickness (RWth) was measured at end diastole as the ratio of (2 × PWth)/LVEDD. Increased RWth was defined as ≥ 0.45.
All echocardiographic measurements were repeated by a second observer (MG) blinded to the values obtained by the first observer (HU). Inter-observer variability was assessed by calculating the coefficient of variation. The coefficient of variation was < 8% for all measurements. Any discrepancy was resolved by consensus. All echocardiographic measurements were repeated 1 week later by an observer (HU) blinded to the results of the previous measurements and intra-observer variability was < 5% for all measurements.
Patterns of LV geometry
Geometric patterns were based on the upper normal limits for LVMI and RWth: (i) normal geometry (NG; normal LVMI and normal RWth); (ii) concentric remodeling (CR; normal LVMI and increased RWth); (iii) concentric hypertrophy (CH; increased LVMI and increased RWth); and (iv) eccentric hypertrophy (EH; increased LVMI and normal RWth) (Citation13).
Doppler echocardiography
Pulsed Doppler ultrasound scanning recordings of the mitral inflow velocities were obtained from the apical four-chamber view by placing the sample volume between the tips of the mitral leaflets (Citation14). Doppler parameters of peak early (E) and late (A) transmitral filling velocities and the ratio of early to late peak velocities (E/A) were analyzed. Doppler ultrasound scanning measurements were calculated from an average of five consecutive cardiac cycles.
Statistical analysis
All analyses were conducted using SPSS 17.0 (SPSS for Windows 17.0, Chicago, IL). Distribution of continuous variables was assessed with the one- sample Kolmogorov Smirnov test. Comparison of categorical variables between the groups was performed using the chi-square test. Analysis of variance (ANOVA) was used in the analysis of continuous variables. A stratified post hoc analyses of echocardiographic, clinical and laboratory variables were performed according to the LV geometric patterns. The associations of hs-cTnT were assessed by the Pearson correlation test. Multivariate backward conditional logistic regression analysis was performed to identify the independent associations of higher level of hs-cTnT. All significant parameters in the bivariate analysis [systolic blood pressure (SBP), diastolic blood pressure (DBP), hs-CRP, serum creatinine level, LVMI, LV geometry and E/A ratio] were selected in the multivariate model. A two-tailed p < 0.05 was considered statistically significant.
Results
In the present study, four different geometric patterns were determined according to LVMI and RWth: (i) 87 patients with NG, (ii) 72 patients with CR, (iii) 56 patients with EH and (iv) 91 patients with CH.
Baseline, laboratory and echocardiographic characteristics
Comparison of baseline, laboratory and echocardiographic characteristics of the groups was demonstrated in . Body mass index (BMI), BSA, SBP and DBP values were different among the groups (p < 0.05, for all). Similarly, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, hs-cTnT, uric acid, creatinine and hs-CRP levels were different among the groups (p < 0.05, for all).
Table I. Comparison of baseline, laboratory and echocardiographic characteristics among the groups.
The highest hs-cTnT concentrations were observed in the CH group compared with the control group and other groups (p < 0.05, for all). Also, hs-cTnT levels of the EH group were higher than the control, NG and CR groups (p < 0.05, for all). However, hs-cTnT levels of the control, NG and CR groups were similar (p > 0.05). Comparison of hs-cTnT levels is shown in .
Figure 1. The relationship between high-sensitivity cardiac troponin T levels and different left ventricle geometry patterns.
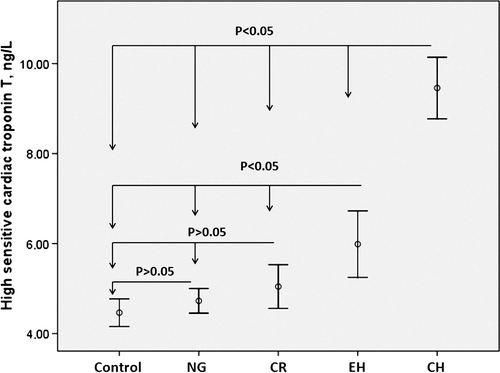
Left atrial diameter (LAD), left ventricle internal diameter (LVID), IVSth, PWth, RWth, LVMI and E/A values were different among the groups (p < 0.05, for all). The measurements of LVMI and RWth were increasing, while the ratio of E/A was decreasing from NG group to the CH group.
Bivariate and multivariate relationships of high-sensitivity cardiac troponin T
Bivariate relationship of hs-cTnT was demonstrated in . hs-cTnT was significantly associated with SBP, DBP, hs-CRP, creatinine, RWth, LVMI, LV geometry and E/A ratio on bivariate analysis. Relationship between hs-cTnT and LVMI was demonstrated in .
Figure 2. The relationship between high-sensitivity cardiac troponin T and left ventricle mass index.
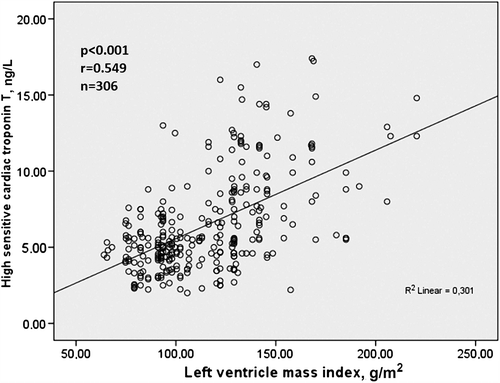
Table II. Bivariate relationship of high-sensitivity cardiac troponin T.
Multivariate logistic regression analysis showed that hs-cTnT was independently associated with LV geometry, LVMI and serum creatinine level ().
Table III. Multivariate logistic regression analysis to identify the independent associations of higher level of high-sensitivity cardiac troponin T.
Discussion
This is the first study that investigated the relationship between hs-cTnT levels and different LV geometry patterns in newly diagnosed hypertensive patients. The main findings of the present study were that: (i) LVMI, RWth and hs-cTnT values were increasing, while the ratio of E/A was decreasing from NG group to the CH group; (ii) In the present study, the highest creatinine value was observed in the CH group; (iii) Our results showed that higher hs-cTnT was independently associated with LV geometry, LVMI and serum creatinine level.
Recent studies have demonstrated that hs-cTnT assays increase the accuracy of diagnosis in the early period of acute myocardial infarction and hs-cTnT allows detection of even minor myocardial necrosis with high precision (Citation1,Citation15). Concerning the measurement of hs-cTnT, large-scale population-based epidemiological studies have shown that multiple factors are associated with chronic, low-grade hs-cTnT elevation (Citation3,Citation4), and thus, increasing age, male gender, HT, diabetes mellitus and reduced renal function have all been shown to be associated with higher hs-cTnT levels in the general population (Citation3,Citation5). Recently, Sato et al. (Citation16) reported that hs-TnT was ≥ 0.003 ng/ml in 78% of patients presenting with essential hypertension (EH) and independently correlated with age, renal function and ECG voltage of hypertrophy. Also, Høiseth et al. showed that arterial HT as well as age and serum creatinine has been independently associated with the level of hs-cTnT on admission for acute exacerbation of chronic obstructive pulmonary disease (Citation17). Also, hs-cTnT level is a novel and useful predictor of future cardiovascular or cerebrovascular events in hypertensive patients (Citation18).
The relationship between LVH and hs-cTnT has been previously demonstrated in hypertensive patients (Citation16). However, the association between LV geometry and hs-cTnT was not investigated in pure hypertensive patients. In the present study, the highest hs-cTnT concentrations were observed in the CH group, followed by those with EH, CR and NG. This finding may be plausible because it has been shown that the CH geometric pattern is associated with a greater risk of hypertensive complications (Citation6,Citation7). A subgroup study of the Framingham heart study (Citation19) demonstrated that patients with CH geometric pattern had the worst prognosis, followed by those with EH, CR and NG. In the LIFE study (Citation20), the prevalence of coronary and cardiovascular disease was almost twice as high in patients with concentric LVH as in the other sub groups. The lowest cardiovascular risk was observed in the group with normal LV geometry (Citation7). Mishra et al. (Citation21) reported that detectable cTnT had a strong association with LVH and LV systolic dysfunction in patients with chronic kidney disease. Also, those authors showed that cTnT is associated with LV geometry. However, in that study, pure hypertensive patients were not included in the study, and the significant factors such as heart failure and diabetes that can affect hs-cTnT levels were not excluded. Therefore, the present study is the first to investigate the relationship between hs-cTnT levels and different LV geometric patterns in pure, newly diagnosed hypertensive patients with preserved EF.
The pathophysiological mechanisms underlying the association between hs-cTnT with LV geometric patterns are still unclear. However, LVH might be responsible for increased hs-cTnT levels in hypertrophic geometric patterns through several mechanisms (Citation8,Citation16). Previous studies and the present study showed that hs-cTnT was independently associated with LVH (Citation8,Citation16). LVH leads to myocardial fibrosis and diastolic dysfunction (Citation22,Citation23). Myocardial fibrosis leads to cardiomyocyte apoptosis and degradation of some molecules (Citation24,Citation25). Moreover, LVH develops in response to chronic pressure and volume overload, which are responsible for cardiomyocyte injury and neurohormonal activation (renin–angiotensin– aldosterone and sympathetic nervous systems) (Citation7,Citation26). In addition, elevated LVM is associated with decreased myocardial flow reserve and reduced tolerance to myocardial ischemia (Citation1,Citation12). On the other hand, increased ischemia may be another reason of higher hs-cTnT levels in hypertrophic geometric patterns. Hickman et al. (Citation27) reported that cardiac troponin may be released by ischemia alone, without necrosis, and they have suggested that the presence of membranous blebs in cardiac myocytes is enabling troponin to be released from cardiac cells due to ischemia alone, without necrosis. The mechanisms mentioned above can be responsible for increasing hs-cTnT levels in hypertensive patients with hypertrophic geometry.
In our study, we found that LVMI, RWth and hs-cTnT values were increasing, while the ratio of E/A was decreasing from NG group to the CH group. The ratio of E/A decreases represents a movement from normal filling pattern to impaired relaxation, and hs-cTnT consequently increases. Previous studies also showed that hs-cTnT is associated with diastolic dysfunction in different disease groups, such as chronic kidney disease, coronary artery disease or congestive heart failure (Citation28,Citation29). Therefore, our results suggest that impairment of diastolic function may be a pathophysiological mechanism responsible for hs-cTnT release in patients with HT.
Patients with HT may exhibit any of the LV geometry patterns. It remains uncertain why some hypertensive patients develop CH and others EH. The major factors that influence the geometric patterns are volume overload, pressure overload and contractile dysfunction. Chronic pressure overload mainly results in CH and chronic volume overload mainly results in EH (Citation13,Citation30). Previously, it has been shown that higher SBP are associated with CH (Citation7). Demographic factors, such as age and gender, diabetes mellitus, neurohormonal activation or coronary artery disease can also modulate the development of different LV geometric pattern (Citation13,Citation30).
Also, in our study, we showed relation between serum creatinine levels and hs-cTnT values in hypertensive patients. The similar relationship had been showed by Høiseth et al. (Citation17). They showed that serum creatinine is independently associated with the level of hs-cTnT in patients on admission for acute exacerbation of chronic obstructive pulmonary disease. In present study, hs-cTnT level was independently associated with creatinine level without chronic kidney disease. The mechanisms of hs-cTnT elevation in patients with renal failure are unclear. Some investigators suggested initially that uremia-induced skeletal myopathy might be the source for pseudo positive hs-cTnT (Citation31). Furthermore, one study showed that TnT is fragmented into molecules small enough to be excreted by the kidney (Citation32). Therefore, the renal clearance might influence the serum concentrations of hs-cTnT without renal failure.
Clinical implication
Elevated hs-cTnT level was independently associated with CH; our findings may have implications for patient assessment. Previous studies showed that the CH geometric pattern is associated with a greater risk of hypertensive complications (Citation6,Citation7) and worst prognosis in hypertensive patients (Citation19). Routine hs-cTnT measurement in these patients may identify high-risk subgroup that warrants echocardiographic evaluation. In addition, our findings suggest that elevations of hs-cTnT concentrations might be an indication for more aggressive treatment.
Study limitations
There were a few limitations in the present study. First, coronary angiography was not performed on the study subjects. However, the patients with a positive effort test, previous coronary artery disease, positive myocardial perfusion scintigraphy, positive history or clinical signs of ischemic heart disease were excluded from our study. Coronary artery disease and myocardial ischemia may be important mechanisms leading to LVH and myocardial damage (Citation33). Subclinical coronary artery disease therefore also may contribute to myocardial damage and circulating hs-cTnT levels in patients with HT. Second, LVH in the majority of patients suggests that the onset of HT is earlier in our patients. Also different LV geometries suggest that duration of HT was different in our patients. Although all patients in this study had newly diagnosed HT, unknown duration of HT is a limitation of the study. Finally, we evaluated LV diastolic function by using transmitral flow PWD recordings. The assessment of LV diastolic function by using tissue Doppler imaging parameters, such as E’, A’ or E/E’, might provide more accurate results.
Conclusions
The present study showed that hs-cTnT levels related with not only LVH but also LV geometry in hypertensive patients. hs-cTnT levels of hypertensive patients increase when LV geometry is progressing from NG to CH geometry. hs-cTnT levels may predict LV geometric patterns with poorer prognosis in hypertensive patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009; 361:858–867.
- Lowbeer C, Gustafsson SA, Seeberger A, Bouvier F, Hulting J. Serum cardiac troponin T in patients hospitalized with heart failure is associated with left ventricular hypertrophy and systolic dysfunction. Scand J Clin Lab Invest. 2004;64:667–676.
- de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. J Am Med Assoc. 2010;304: 2503–2512.
- Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123: 1367–76.
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50: 2173–95.
- Koren M J, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352.
- Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558.
- Hoshide S, Fukutomi M, Eguchi K, Watanabe T, Kabutoya T, Kario K. Clinical and Change in high-sensitive cardiac troponin T on hypertensive treatment. Exp Hypertens. 2013;35:40–44.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al.; Chamber Quantification Writing Group. Recommendations for Chamber Quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Friehs I, del Nido PJ. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg. 2003; 75:S678–684.
- Negri F, Sala C, Re A, Mancia G, Cuspidi C. Left ventricular geometry and diastolic function in the hypertensive heart: Impact of age. Blood Press. 2013;22:1–8.
- Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: New insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12: 426–440.
- Chenevier-Gobeaux C, Meune C, Freund Y, Wahbi K, Claessens YE, Doumenc B, et al. Influence of age and renal function on high-sensitivity cardiac troponin T diagnostic accuracy for the diagnosis of acute myocardial infarction. Am J Cardiol. 2013 doi:pii: S0002-9149(13)00627-9. 10.1016/j.amjcard.2013.02.024.
- Sato Y , Yamamoto E , Sawa T, Toda K, Hara T, Iwasaki T, et al. High-sensitivity cardiac troponin T in essential Hypertension. J Cardiol. 2011;58:226–231.
- Høiseth AD, Omland T, Hagve T, Brekke PH, Søyseth V. Determinants of high-sensitivity cardiac troponin T during acute exacerbation of chronic obstructive pulmonary disease: A prospective cohort study. BMC Pulmonary Med. 2012; 12:22.
- Setsuta K, Kitahara Y, Arae M, Ohbayashi T, Seino Y, Mizuno K. Elevated cardiac troponin T predicts adverse outcomes in hypertensive patients. Int Heart J. 2011;52: 164–169.
- Krumolz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–84.
- Dahlöf B, Devereux R, de Faire U, Fyhrquist F, Hedner T, Ibsen H, et al. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: Rationale, design, and methods. The LIFE Study Group. Am J Hypertens. 1997;10:705–13.
- Mishra RK, Li Y, DeFilippi C, Fischer MJ, Yang W, Keane M, et al. Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013; 61:701–709.
- Krauser DG, Devereux RB. Ventricular hypertrophy and hypertension: Prognostic elements and implications for management. Herz. 2006;31:305–316.
- Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517.
- González A, Fortuño MA, Querejeta R, Ravassa S, López B, López N, et al. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–562.
- Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–272.
- Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: Correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–178.
- Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, et al. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010;411: 318–323.
- Bakal RB, Hatipoglu S, Kahveci G, Omaygenc MO, Unkun T, Akgun T, et al. Determinants of high sensitivity troponin T concentration in chronic stable patients with heart failure: Ischemic heart failure versus non-ischemic dilated cardiomyopathy. Cardiol J. 2013 Jun 25. doi: 10.5603/CJ.a2013. 0061.
- Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Kikumoto Y, et al. Serum high-sensitivity cardiac troponin T is a significant biomarker of left-ventricular diastolic dysfunction in subjects with non-diabetic chronic kidney disease. Nephron Extra. 2011;1:166–77. doi: 10.1159/ 000333801. Epub 2011 Oct 25.
- Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792. Review.
- Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: A meta-analysis. Circulation. 2005;112:3088–3096.
- Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–5.
- Solaro RJ. Troponin I, stunning, hypertrophy, and failure of the heart. Circ Res. 1999;84:122–124.
