Abstract
Objective. Our aim was to investigate retinal nerve fiber layer (RNFL) thickness in hypertensive patients using spectral-domain optical coherence tomography (SD-OCT) and to evaluate the relationship between RNFL thickness and carotid intima media thickness (CIMT). Methods. This study included 59 patients with hypertension (HT) (53.6 ± 10.7 years) and 54 age-matched healthy controls (51.0 ± 8.1 years). We evaluated anthropometric and metabolic parameters as well as RNFL and CIMT measurements in patients with hypertension and controls. Results. The average RNFL thickness was 86.60 ± 10.86 μm in hypertensive patients and 93.63 ± 7.30 μm in healthy controls (p < 0.001). Selective thinning of the RNFL was found in the superior and inferior quadrants. Mean CIMT values were higher in patients with HT (0.80 ± 0.15 mm) than the healthy subjects (0.71 ± 0.1 mm) (p < 0.001). The average, inferior and nasal RNFL thickness were negatively associated with diastolic blood pressure respectively (r = − 0.112, r = − 0.210, r = − 0.225). There was an inverse correlation between RNFL thickness in the average and superior retinal quadrant and CIMT (r = − 0.201, r = − 0.185). There were no correlations between RNFL thickness and age, body mass index, fasting plasma glucose, lipid parameters, high-sensitive C-reactive protein and microalbuminuria. Conclusion. RNFL thickness is reduced in hypertensive patients and may be associated with atherosclerosis.
Introduction
Hypertension is an established risk factor of atherosclerosis, and associated with a higher risk of death and ischemic events (Citation1). It is important to identify patients with hypertension at risk of developing cardiovascular events. Carotid intima media thickness (CIMT) is a validated parameter for detecting subclinical atherosclerosis (Citation2,Citation3). In numerous studies, CIMT was shown to predict the occurrence of both stroke and myocardial infarction, independently of traditional cardiovascular risk factors (Citation4–7).
Hypertension also has a high association with an increased risk of retinal microvascular abnormalities, such as retinal arteriolar narrowing and retinopathy (Citation8–10). Retinal arteriolar caliber was shown previously to be related inversely to blood pressure (BP) levels (Citation11). In some studies, retinal microvascular disorders were found to be associated with cardiovascular disease and mortality (Citation12–15). Recently, it was demonstrated that pulse-wave velocity of optic nerve head circulation was correlated with brachial–ankle pulse-wave velocity and CIMT (Citation16).
Evaluation of the retinal nerve fiber layer (RNFL) has been a well-established clinical and investigational tool (Citation17). The retinal nerve fibers occur in the innermost layer of the retina and are formed by ganglion cell axons, which react to impulses from the photoreceptors to the central vision by way of the optic nerve. Axon damage is characterized by thinning of the RNFL. RNFL thickness can be affected in numerous neurodegenerative diseases (Citation18,Citation19). Optical coherence tomography (OCT) can be used for this purpose via obtaining histological sections of the retina and RNFL thickness measurements (Citation20).
Hypertension plays an important role in the development of ischemic disorders of retina and the other systemic risk factors are diabetes mellitus, ischemic cardiovascular disease, hyperlipidemia and atherosclerosis (Citation21). As diabetes mellitus induces the ischemia of retina, it results in the thinning of the RNFL (Citation22). Numerous studies have also demonstrated the association between thinning of the RNFL and diabetes mellitus (Citation23–26). However, RNFL thicknesses have not yet been evaluated in systemic hypertension.
The aim of the current study was to evaluate the impact of systemic hypertension on RNFL thickness using spectral-domain OCT (SD-OCT). Our second aim was to investigate the relationship between RNFL thickness and CIMT.
Materials and Methods
Study population
Fifty-nine patients with essential hypertension (mean age: 53.62 ± 10.7 years) and age-matched 54 healthy subjects (mean age: 51.0 ± 8.18 years) were enrolled in the current study. All the participants gave a written consent. The study protocol was approved by the local ethical committee.
Arterial BP measurements were performed by the same calibrated sphygmomanometer after 5 min of rest in a sitting position; measurements were performed on both arms at 5-min intervals. The average of the two measurements obtained in each arm was taken as the subject's BP. Patients with BP values greater than 140/90 mmHg were considered hypertensive (Citation3). Newly diagnosed hypertensive patients were excluded from the study. We included patients with essential hypertension who were receiving anti-hypertensive drugs at least for 1 year. The mean duration of hypertension was 6.7 years (1–30 years) − 40.6% of the patients were treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 25.4% were receiving calcium channel blockers, 28.8% of the patients were using beta-blockers and 50.8% of them were treated with diuretics; 56.1% of the patients were treated with monotherapy, 23.7% of them were treated with two drugs and 20.2% of them were treated with three or more drugs. Almost half (45.6%) of the patients were poorly treated.
Subjects with glaucoma (diagnosis was based on cup-to-disc ratio > 50%, cup-to-disc ratio asymmetry between two eyes > 20%, corrected intraocular pressure > 21 mmHg and glaucomatous visual field defects), pseudoexfoliation syndrome, high myopia and hypermetropia (greater than 6.0 diopters), anomalous optic disc, age-related macular degeneration, macular edema (macular thickness measured by optic coherence tomography greater than 300 μm), peripheral vasospasm, family history of glaucoma, history of ocular trauma, optic neuropathy, use of corticosteroid and glaucoma medications, diabetes mellitus, chronic kidney disease and a previous ischemic event were excluded from the study.
Clinical assessment and biochemical measurements
Weight, height and waist circumference were measured. The body mass index (BMI) was calculated by dividing the body weight in kilograms by the square of the height in meters (kg/m2). Waist circumference (WC) was measured at the narrowest level between the costal margin and iliac crest.
Venous blood samples were obtained from all subjects following an 8–12-h overnight fast. The levels of fasting plasma glucose (FPG), total cholesterol, triglyceride (TG), high-density lipoprotein-cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), high-sensitive C-reactive protein (hs-CRP), urea, creatinine and microalbuminuria were measured.
The hexokinase method was used to measure glucose levels, and photometric method (Abbott Architect c16000 autoanalyzer) was used to measure the total cholesterol, TG, HDL- and LDL-cholesterol levels. The concentration of hsCRP was measured using immunotubidimetric method with Abbott Architect C16000 autoanalyzer (Abbott Diagnostic, USA). The cut-off for hsCRP was taken as < 0.5 mg/dl.
Urinary albumin excretion (as an indicator of nephropathy) was measured in an early morning urine sample as albumin-to-creatinine ratio using a Cobas Integra 800 (Roche Diagnostics, Mannheim, Germany). Micro- or macro-albuminuria was present if urinary albumin excretion in at least two of three consecutive urine samples, 2 months apart, was 30–299 mg/g creatinine or ≥ 300 mg/g creatinine, respectively (Citation27).
Measurement of retinal fiber layer thickness
All patients and controls underwent ophthalmologic testing, including assessment of visual acuity and color vision, slit-lamp examination with intraocular pressure measurement, visual fields with automated perimetry (Octopus 900; Haag Streit, Koeniz, Switzerland) and funduscopy. OCT imaging was performed with the Cirrus HD SD-OCT (Carl Zeiss Meditec, Dublin, CA). The OCT imaging was performed with the participants’ pupils dilated to obtain higher signal quality. Scans that had signal strengths lower than 8 were not included in the study to obtain images with minimal measurement errors. Average temporal, nasal, inferior, superior quadrant peripapillary RNFL thicknesses were obtained from the OCT with optic disc 200 × 200 cube scan protocol along a circle with a diameter of 3.46 mm around the center of the optic disc. OCT imaging was performed by the same ophthalmologist blinded to the patients.
Measurement of carotid intima media thickness
Ultrasonography was performed on all participants using a high-resolution ultrasonography scanner (Aplio 500, Toshiba Medical Systems, Tokyo, Japan) with a PLT-805AT linear array transducer. Measurements were performed on the right and left carotid arteries. The patient was lying supine with the head directed away from the side of interest and the neck slightly extended. The transducer was manipulated so that the near and far walls of the common carotid artery (CCA) were parallel, and the lumen diameter was maximized in the longitudinal plane. The region 1 cm proximal to the carotid bifurcation was identified, and the CIMT of the far wall was evaluated as the distance between the lumen–intima interface and the media–adventitia interface. The CIMT was measured on a frozen frame of a suitable longitudinal image, with the image magnified to achieve a higher resolution of detail. The CIMT measurement was obtained from four contiguous sites at 1-mm intervals on each carotid artery, and the average of all eight measurements was used for analysis. All measurements were performed by the same cardiologist, who was blinded to patient data (Citation28).
Statistical analysis
Data were analyzed using SPSS Software (Version 17, SPSS, Inc., Chicago, IL, USA). Results were expressed as mean± standard deviation. The Mann–Whitney U test was used to compare the continuous variables and the chi-square test was used to compare categorical variables. Spearman's rank correlation test was used for calculation of associations between variables. Multiple regression analyses were performed to identify the independent determinants for RNFL thickness. A p-value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics of the subjects
The demographic and clinical characteristics of the study population are shown in . There were no significant differences in age, sex, BMI and waist circumference between patients with hypertension and healthy controls. Serum levels of FPG were higher in patients with hypertension (100.13 ± 9.44 mg/dl) compared with the healthy subjects (95.71 ± 7.09 mg/dl) (p = 0.006). Among the lipid parameters, total-cholesterol, LDL-cholesterol and TG levels were significantly higher in the hypertensive group in comparison with the controls, respectively (p = 0.002, p = 0.018, p = 0.028). Although serum urea and creatinine levels were in normal range in all patients with hypertension, values of urea were higher compared with those in healthy subjects (p = 0.039). Serum levels of uric acid and hsCRP were also higher in the patient group in comparison with the control group (). Mean CIMT values were higher in patients with hypertension (0.80 ± 0.15 mm) than the healthy subjects (0.71 ± 0.10 mm) (p = 0.001).
Table I. Comparison of the clinical and biochemical parameters in patients with hypertension and control group.
Comparison of RNFL thickness between the groups
According to quadrants, the mean RNFL thickness of the two groups is shown in . The average RNFL thickness was 86.64 ± 10.86 μm in hypertensive patients and 93.63 ± 7.30 μm in healthy controls (p < 0.001). Selective thinning of the RNFL was found in the superior and inferior quadrants (). Mean superior RNFL thickness was 106.98 ± 17.51 μm in patients with hypertension and 118.91 ± 14.74 μm in controls (p < 0.001). Mean inferior RNFL thickness values were lower in hypertensive patients (109.2 ± 18.69 μm) compared with the control group (118.59 ± 13.81 μm) (p = 0.003). The RNFL thickness did not differ significantly between patients with hypertension and controls in temporal and nasal quadrants respectively (p = 0.401, p = 0.150).
Table II. Retinal nerve fiber layer thickness in patients with hypertension and healthy controls.
The correlations between RNFL thickness, CIMT and cardiometabolic parameters
The average (r = − 0.112, p = 0.038), inferior (r = − 0.210, p = 0.026) and nasal (r = − 0.225, p = 0.016) RNFL thickness were negatively associated with diastolic BP. There was also an inverse correlation between RNFL thickness in the average and superior retinal quadrant and CIMT (r = − 0.201, r = − 0.185) (). The negative correlation between average RNFL thickness and CIMT is shown in . There were no correlations between RNFL thickness and age, BMI, FPG, lipid parameters, hsCRP and microalbuminuria.
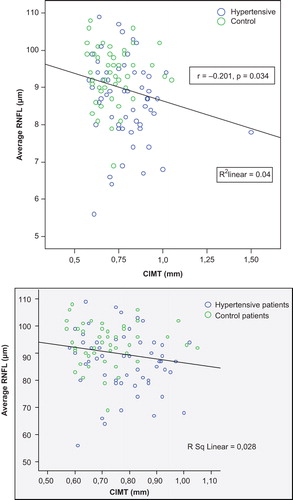
Table III. The correlation between retinal nerve fiber layer thickness, carotid intima media thickness (CIMT) and cardiometabolic parameters in the study population.
Multiple linear regression analyses
We performed a multiple linear regression analyses including RNFL thickness as dependent variable and age, uric acid, systolic BP, diastolic BP, LDL-cholesterol, TG, hsCRP and CIMT as explanatory variables. In this analysis, we did not find any significant and independent predictor for RNFL thickness.
Discussion
Our study showed that RNFL of hypertensive patients was thinner than healthy controls and this difference was most prominent in the superior and inferior quadrants. Furthermore, average and superior RNFL thickness was inversely associated with CIMT.
Blood supply of retina originates from choroidal arteries, posterior ciliary arteries and in part from pial arteries networks. Thinning of the RNFL was demonstrated in patients with migraine (Citation29). Associated with neurovascular pathogenesis of migraine, vasoconstriction in particular during the migraine attacks affects retrobulbar artery. Consequently, due to this effect, retinal hypoperfusion and retinal anomalies related to ganglionic retinal cell death occur.
Arterial hypertension can influence the retina blood flow in a number of ways. Increased vascular resistance in the terminal arterioles can derange retina blood supply. Secondary hypertensive vascular changes also occur in retina vessels, and finally blood flow autoregulation is disturbed (Citation21). As a result, these changes make the optic nerve prone to perfusion deficiency in hypertension and retinal ischemia can develop. In the current study, we hypothesized whether RNFL thickness may be reduced in patients with systemic hypertension.
RNFL thinning has been described in patients with type 2 diabetes mellitus in a number of studies (Citation23–26). However, it has not yet been evaluated in hypertension. Systemic hypertension is a systemic condition and affects the optic nerve vascular circulation. In the present study, we showed that RNFL of hypertensive patients was thinner than healthy controls. The average, inferior and nasal RNFL thickness were negatively associated with diastolic BP. Thinning of the RNFL was found to be more prominent in the superior quadrant of retina in diabetic patients in most of the studies (Citation24,Citation30). In our study, the superior and inferior quadrants of RNFL were thinner in patients with hypertension. We suggest that particularly superior and inferior quadrants of retinal nerve are affected in systemic hypertension.
Atherosclerotic changes in the large arteries may play a role in the development of ischemic disorders of the retina (Citation31). Inflammation and endothelial dysfunction play a role in the pathogenesis of both large atherosclerosis and retinal microvascular diseases (Citation32). Retinal vascular abnormalities were found to be related to various markers of atherosclerosis in a number of studies (Citation33–36). Carotid intima-media thickness is a surrogate marker of atherosclerosis. Lim et al. demonstrated the upper limit of carotid IMT in healthy population (Citation37). In our study, the control group had similar values of CIMT to the results from the study of Lim et al. We also found higher CIMT values in patients with hypertension than the control group.
In the present study, we hypothesized that carotid atherosclerosis may also be associated with thinning of RNFL. Recently, we have shown a negative association between RNFL thinning and carotid atherosclerosis in patients with type 2 diabetes mellitus (Citation38). We also found that there was a negative correlation between RNFL thickness and CIMT in the current study. Hayreh & Jonas showed reduced RNFL of rhesus monkeys in chronic experimental arterial hypertension and atherosclerosis (Citation39). Our data provide support for the hypothesis that systemic atherosclerosis may be associated with thinning of RNFL in patients with systemic hypertension. Additionally, further research is needed to understand the link between atherosclerosis and RNFL thinning.
Retinal examination is recommended as part of the routine assessment of people with hypertension and its value has been questioned, partly because clinical ophthalmoscopy is highly unreliable for detecting retinal microvascular lesions (Citation40). SD-OCT can provide B-scan images of the retina with a resolution of 8–10 μm. Thus, the thickness of RNFL can be measured reliably and accurately by the installed software.
In conclusion, our data suggest that retinal fiber layer thickness is reduced in hypertensive patients and may be associated with atherosclerosis.
Acknowledgments
The authors declare no competing interests. No financial support was received for this paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A metaanalysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913.
- Stein JH, Fraizer MC, Aeschlimann SE, Nelson-Worel J, McBride PE, Douglas PS. Vascular age: Integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27:388–392.
- 2013 ESH/ESC Guidelines for the management of arterial hypertension. Rev Esp Cardiol (Engl Ed). 2013;66:880.
- Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, TorpPedersen C, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–891.
- Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96:1432–1437.
- O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22.
- Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607.
- Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, et al.; Atherosclerosis Risk in Communities Study. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255.
- Wong TY, Klein R, Klein BE, Tielsch, JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2011;46:59–80.
- Wong TY, Shankar A, Klein R, Klein BE and Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. Br Med J. 2004;329:79.
- Gepstein R, Rosman Y, Rechtman E, Koren-Morag N, Segev S, Assia E, et al. Association of retinal microvascular caliber with blood pressure levels. Blood Press. 2012;21:191–196.
- Sharrett, AR, Hubbard LD, Cooper LS, Sorlie PD, Brother RJ, Nieto FJ, et al. Retinal arteriolar diameters and elevated blood pressure: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270.
- Wong, TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140.
- Van Hecke MV, Dekker JM, Nijpels G, Moll AC, Van Leiden HA, Heine RJ, et al: The Hoorn Study. Retinopathy is associated with cardiovascular and all-cause mortality in both diabetic and nondiabetic subjects: The Hoorn Study. Diabetes Care. 2003;26:2958.
- Wong, TY, Klein R, Sharrett, AR, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women: The Atherosclerosis Risk In Communities Study. JAMA. 2002; 287:1153–1159.
- Shiba T, Takahashi M, Hori Y, Maeno T, Shirai K. Optic nerve head circulation determined by pulse wave analysis is significantly correlated with cardio ankle vascular index, left ventricular diastolic function, and age. J Atheroscler Thromb. 2012;19:999–1005.
- Skarf B. Retinal nerve fibre layer loss in diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86:709.
- Pueyo V, Ara JR, Almarcegui C, Martin J, Güerri N, García E, et al. Subclinical atrophy of the retinal nerve fiber layer in multiple sclerosis. Acta Ophthalmol. 2013;88:748–752.
- Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuro-Ophthalmol. 2013;33:58–61.
- Nassif N, Cense B, Park B, Pierce M, Yun S, Bouma B. In vivo high resolution video-rate spectral domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12:367–376.
- Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009;28:34–62.
- Oshitari T, Roy S. Diabetes: A potential enhancer of retinal injury in rat retinas. Neurosci Lett. 2005;390:25–30.
- Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye (Lond). 2009;23:884–889.
- Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385.
- Park HY, Kim IT, Park CK. Early diabetic changes in the nerve fibre layer at the macula detected by spectral domain optical coherence tomography. Br J Ophthalmol. 2011;95:1223–1228.
- Shahidi AM, Sampson GP, Pritchard N, Edwards K, Vagenas D, Russell AW, et al. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabet Med. 2012;29:e106–e111.
- Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al.;National Kidney Disease Education Program–IFCC Working Group on Standardization of Albumin in Urine. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al.; American Society of Echocardiography Carotid Intima-Media Thickness Task Force.Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111.
- Kirbas S, Tufekci A, Turkyilmaz K, Kirbas A, Oner V, Durmus M. Evaluation of the retinal changes in patients with chronic migraine. Acta Neurol Belg. 2013;113:167–172.
- Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86:725–728.
- Hayreh SS. Retinal and optic nerve head ischemic disorders and atherosclerosis: Role of serotonin. Prog Retin Eye Res. 1999;18:191–221.
- Wong TY, McIntosh R. Systemic associations of retinal microvascular signs: A review of recent population-based studies. Ophthalmic Physiol Opt. 2005;25:195–204.
- Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20: 1644–1650.
- Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The Cardiovascular Health Study. Ophthalmology. 2003;110:658–666.
- Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, et al.; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: The atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–1234.
- Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, Gottdiener JS, et al. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br J Ophthalmol. 2002;86: 84–90.
- Lim TK, Lim E, Dwivedi G, Kooner J, Senior R. Normal value of carotid intima-media thickness—a surrogate marker of atherosclerosis: Quantitative assessment by B-mode carotid ultrasound. J Am Soc Echocardiogr. 2008;21:112–116.
- Sahin SB, Sahin OZ, Ayaz T, Karadag Z, Türkyılmaz K, Aktas E, et al. The relationship between retinal nerve fiber layer thickness and carotid intima media thickness in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014 Oct 2. pii: S0168-8227(14)00410-0. doi: 10.1016/j.diabres.2014.09.010.
- Hayreh SS, Jonas JB. Appearance of the optic disk and retinal nerve fiber layer in atherosclerosis and arterial hypertension: An experimental study in rhesus monkeys. Am J Ophthalmol. 2000;130:91–96.
- Klein R. Retinopathy in a population-based study. Trans Am Ophthalmol Soc. 1992;90:561–594.
