Abstract
The SYMPLICITY studies showed that renal denervation (RDN) is feasible as novel treatment for resistant hypertension. However, RDN is a costly and invasive procedure, the long-term efficacy and safety of which has not yet been proven. Therefore, we designed the INSPiRED trial to compare the blood pressure lowering efficacy and safety of RDN vs usual medical therapy. INSPiRED is a randomized controlled trial enrolling 240 treatment-resistant hypertensive patients at 16 expert hypertension centres in Belgium. Eligible patients, aged 20–69 years old, have a 24-h ambulatory blood pressure of 130 mmHg systolic or 80 mmHg diastolic or more, while taking at least three antihypertensive drugs. They are randomized to RDN (EnligHTNTM, SJM system) plus usual care (intervention group) or usual care alone (control group) in a ratio of 1:1. The primary endpoints for efficacy and safety, measured after 6 months, are the baseline-adjusted between-group differences in 24h systolic blood pressure and in glomerular filtration rate as estimated by the Chronic Kidney Disease Epidemiology Collaboration equation. Follow-up will continue up to 36 months after randomization. INSPiRED is powered to demonstrate a 10-mmHg difference in systolic blood pressure between randomized groups with a two-sided p-value of 0.01 and 90% power. It will generate long-term efficacy and safety data, identify the subset of treatment-resistant hypertensive patients responsive to RDN, provide information on cost-effectiveness, and by doing so INSPiRED will inform guideline committees and health policy makers.
Trial registration: ClinicalTrials.gov identifier: NCT01505010.
Introduction and rationale
Resistant hypertension is a blood pressure that remains above goal in spite of the concomitant use of three or more antihypertensive drugs from different classes. Depending on populations studied and methods applied, the prevalence of treatment-resistant hypertension varies from 10% to 15% (Citation1,Citation2). Renal sympathetic nerves contribute to the development and perpetuation of hypertension (Citation3). Surgical thoracic sympathectomy and splanchnicectomy have been used in the past to reduce blood pressure. However, they were abandoned because of high morbidity and major adverse effects. Recently, catheter-based renal denervation (RDN) emerged as a potential treatment modality for resistant hypertension (Citation4,Citation5).
The SYMPLICITY studies (Citation4,Citation5) showed that RDN was feasible in treating resistant hypertension. However, several issues remained unaddressed, which have been reviewed in detail elsewhere (Citation6,Citation7). The subsequently published literature includes several observational studies and small series of cases. As summarized by a recent meta-analysis (Citation8), among 23 studies investigating RDN in resistant hypertensive patients, only two (Citation5,Citation9) were randomized controlled trials. One trial (Citation9) was of low quality (Citation10) and did not meet the CONSORT criteria (Citation11). Three reports were case–control studies and all other studies were single-arm case studies without a control group. Therefore, the current evidence for RDN rests on only one randomized controlled trial (Citation5). These limitations highlight the need for well-conducted randomized controlled trials to evaluate the long-term efficacy and safety of RDN in truly resistant hypertension patients. We therefore designed the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug- Resistant Hypertension (INSPiRED).
Design
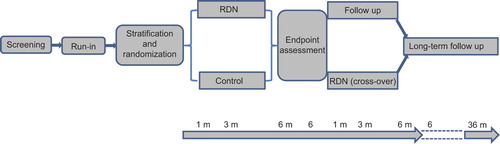
INSPiRED has a multicentre randomized controlled design. Eligible patients will be recruited at 16 tertiary referral centres in Belgium and randomized to usual medical treatment (control group) or usual medical treatment plus RDN (intervention group). The trial consists of six stages (): (i) a screening visit, at which the patient's eligibility will be checked according to inclusion and exclusion criteria, and informed consent will be obtained. (ii) A run-in period, lasting 1–3 months to allow investigators to optimize medical treatment. During the run-in period, additional examinations will be performed to exclude secondary hypertension. Office, home and the 24-h ambulatory blood pressures will be recorded. The measurements obtained at the end of the run-in period will serve as baseline. (iii) Stratification and randomization: eligible patients will be stratified by centre and age group (20–49 vs 50–69), and subsequently randomized in a one- to-one proportion to the control or intervention group. (iv) RDN procedure: patients randomized to the intervention group will undergo RDN shortly after randomization, while continuing their antihypertensive medications with dose adjustments if required; in the control group, patients will be maintained on optimized drug treatment. Six months after having been randomized, patients of the control group can cross over and undergo RDN. (v) Follow-up at the participating centres will be scheduled at 1, 3 and 6 months after randomization (). If patients of the control group cross over, they will be followed up at the same interval up to 6 months after the procedure. A visit 2 months after RDN is optional. (vi) Long-term supervised or non-supervised follow-up will extend up to 36 months with visits planned at 6 monthly or annual intervals. Supervised follow-up refers to patients attending the INSPiRED clinical centres. Investigators will continue to collect information in patients not being followed up at the INSPiRED centres, so called non-supervised follow-up.
Figure 1. Schematic representation of the INSPiRED trial. During the run-in period, antihypertensive drug treatment is optimized and secondary hypertension is excluded. Patients are randomized to renal denervation (RDN) or control. After randomization, optimized drug treatment is continued in both treatment arms, but can be adjusted according to the achieved blood pressure level. After assessment of the endpoints at 6 months, control patients can cross over to renal denervation. Follow-up visits are scheduled at 1, 3 and 6 months after randomization and at 1, 3 and 6 months after renal denervation in control patients crossing over with a visit at 2 months being optional. The long-term follow-up last up to 36 months after randomization.
On 31 January 2014, the Ethics Committee of the Faculty of Medicine of the University of Leuven approved INSPiRED. The trial will start with a pilot phase at two centres (Cliniques Universitaires Saint-Luc, Bruxelles and University Hospital Leuven). The pilot phase aims to assess feasibility in terms of recruitment rate as a proportion of screened patients, to test the procedures as described in the protocol, and to evaluate the 1-month responses in 24-h ambulatory blood pressure in 16 randomized patients, of whom eight (four at each centre) will undergo RDN and eight (four at each centre) will be randomly assigned to medical treatment. The results of this pilot trial will be forwarded to the Ethics Committees of the two centres. The expectation is that the INSPiRED protocol as piloted will roll over in the full trial without major changes.
Primary and secondary outcomes
The primary endpoints for efficacy and safety are the baseline-adjusted between-group differences in 24-h systolic blood pressure and in estimated glomerular filtration rate (eGFR), as estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Citation12). To compare changes in renal function with other studies, such as the SYMPLICITY trials (Citation4,Citation5), eGFR will also be computed by the Modification of Diet in Renal Disease (MDRD) formula (Citation13). Secondary endpoints for efficacy include the proportion of patients reaching and maintaining blood pressure control, defined as a 24-h blood pressure level below 130 mmHg systolic and 80 mmHg diastolic, a daytime blood pressure below 135 mmHg systolic and 85 mmHg, a self-measured blood pressure at home below 135 mmHg systolic and 85 mmHg, or an office blood pressure below 140 mmHg systolic and 90 mmHg diastolic. Secondary endpoints for safety include acute and chronic procedural safety, a decline in eGFR by 25% or more, a new renal arterial stenosis of over 60% confirmed by renal angiogram at 6 months, as well as cardiovascular outcomes. Other secondary endpoints include the intensity of medical treatment, quality of life assessment and the cost-effectiveness analysis.
Sample size
To detect a 10-mmHg difference in systolic blood pressure between the randomized groups with the standard deviation set at 20 mmHg and a two-sided p-value of 0.01 and 90% power, 480 patients would have to be screened and 240 randomized, assuming 50% screening failures. Assuming a baseline-adjusted between-group difference of 10 ml/min/1.73 m2 in eGFR and a standard deviation of 20 ml/min/1.73 m2, 100 patients randomized per group should be sufficient to reach 0.05 significance with 95% power.
Selection of patients
The inclusion and exclusion criteria are listed in . Eligible patients are 20–69 years old and have essential hypertension. The 24-h ambulatory blood pressure should be 130 mmHg systolic or 80 mmHg diastolic or higher, while the patient is taking three or more antihypertensive medications from different classes, preferably including a diuretic (Citation14). An aldosterone receptor antagonist and/or a betablocker should at least have been tried, unless contra-indicated.
Table I. Criteria for patient selection in the INSPiRED trial.
Renal denervation
RDN will be performed using the EnligHTNTM multi-electrode denervation system (St Jude Medical, St. Paul, Minnesota, USA). This system allows four ablations to be performed simultaneously. One 60-s ablation will be delivered at the mid/distal segment of the renal artery with activation of the four electrodes making up the basket. In case of a long renal artery, a more proximal positioning of the basket will allow a second ablation of 60 s with activation of all or part of the electrodes. The same RDN procedure will be performed at the main renal arteries of either side. Angiography will be performed to control the renal arterial anatomy after the procedure and to identify potential spasm between and after ablations.
Follow-up
Patients will be followed up to 6 months after randomization () for assessment of the primary and secondary endpoints. Long-term supervised or non-supervised follow-up will continue up to 36 months after RDN to evaluate the long-term safety and efficacy of the procedure and to compare the incidence of events between randomized groups.
Ancillary studies
The INSPiRED study will offer the possibility to set up optional ancillary studies with a longitudinal design comparing the influence of randomized treatment on various organ systems. A bio-bank of frozen serum and plasma samples obtained at different time points of the trial will be set up at the Studies Coordinating Centre (SCC) in Leuven. All centres will perform echocardiography as part of routine clinical care. However, INSPiRED centres can also opt to participate in an ancillary echocardiographic study, using the protocols as implemented in FLEMish study on ENVironment Genes and Health Outcomes (FLEMENGHO) and in the European Project of Genes in Hypertension (EPOGH) (Citation15,Citation16). Structure and function of the large arteries, the microcirculation and endothelial function can also be evaluated, following the standard operational procedures implemented in the FLEMENGHO and EPOGH studies (Citation17).
Management of hypertension
Antihypertensive treatment
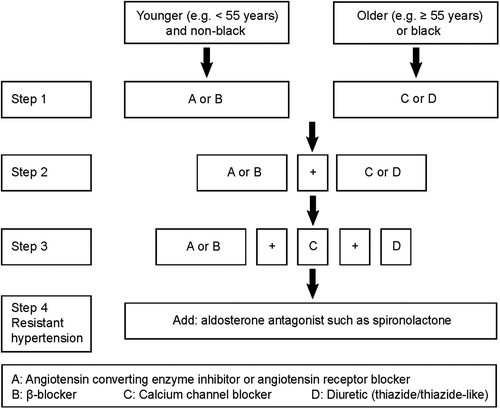
To optimize blood pressure lowering drug treatment, the investigators will follow current guidelines (Citation18,Citation19). Treatment strategy will be standardized as follows: (i) by using combinations of antihypertensive drugs with different mode of action in line with the AB/CD algorithm (Citation18), as illustrated in ; (ii) by using antihypertensive agents with a long duration of action, so-called forgiving drugs (Citation20); (iii) by enhancing adherence via reduction of the pill load and by prescription of single-pill combination tablets, including two or three antihypertensive agents in varying doses; (iv) for each antihypertensive drug, the highest dose that does not produce side-effects will be prescribed; (v) antihypertensive drug combinations should preferably include a diuretic; (vi) if not contra-indicated, aldosterone receptor antagonists and betablockers will at least be attempted. After RDN, blood pressure lowering drug treatment will be maintained at an intensity required to keep the patient's blood pressure within or as close as possible to the normotensive range (Citation18,Citation19,Citation21).
Figure 2. INSPiRED algorithm for adjustment if antihypertensive drug treatment. Recommendation for combining blood-pressure lowering drugs according to the ABCD rule. Modified from: JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, The Stroke Association. Heart 2005;91: supplement 5:v1–52. Copyright © 2005 BMJ. Reproduced with permission from BMJ Publishing Group Ltd.
Lifestyle
Lifestyle changes will be recommended and reinforced during the whole trial, including stopping smoking, moderation of excessive alcohol intake, regular physical activity, weight reduction in overweight or obese patients, dietary measures to control dyslipidaemia, and a moderation of salt intake (Citation22).
Measurements
lists measurements to be obtained by visit.
Table II. Measurements by visit.
Blood pressure
In INSPiRED, the clinic blood pressure, self-measured home blood pressure and 24-h ambulatory blood pressure will be measured according to the recommendations of the European Society of Hypertension (Citation19,Citation23), using validated devices. If at the screening visit systolic and diastolic blood pressure differences between the left and right arm are less than 10 mmHg, the investigator will select the non-dominant arm for all blood pressure measurements throughout the study. Otherwise, the arm giving the highest blood pressure readings must be chosen. A standard cuff of 22 × 12 cm will be used if the arm circumference is less than 32 cm. For arms with a larger circumference, cuffs with a bladder size of at least 31 × 15 cm will be used.
Clinic blood pressure will be measured after the patient has been seated for 5 min in a quiet room. Three consecutive blood pressure readings at intervals of 30–60 s will be obtained while the patient remains in the sitting position. The investigator will then ask the patient to stand up and obtain two additional blood pressure readings. The 24-h ambulatory blood pressure recordings should include at least 20 readings for the daytime or awake blood pressure and seven readings for the night-time or asleep blood pressure (Citation23). The whole recording should cover at least 20 h with no interval between two successive reading longer than 3 h. On the day of the recording, the patients must complete a diary card, from which it should be possible to retrieve the timing of meals and intake of drugs, and when sleep at night started and ended. Self-measurement of blood pressure at home will be done according to current guidelines (Citation24,Citation25) using properly validated devices. The investigators will educate patients in the proper use of the home monitors. Patients should complete a diary providing information on the timing and values of blood pressure and heart rate. Patient will be requested to obtain two consecutive blood pressure readings in the sitting position, twice daily, in the morning between 06:00 and 10:00 h before breakfast and intake of medications, and in the evening between 18:00 and 22:00 h, during the 7 days preceding each clinical visit. The self-measured home blood pressure is the average of all blood pressure readings of the last six days, discarding the readings of the first day (Citation25).
Adherence
In the INSPiRED trial, several instruments will be implemented to assess adherence. A simple questionnaire with eight questions (Citation26) will be used to assess adherence to antihypertensive drugs. Jung and co-workers (Citation27) developed a liquid chromatography– mass spectrometric method that allows all antihypertensive drugs or their metabolites in a single urine sample to be detected in a qualitative manner. This approach will be mandatory in INSPiRED. A urine sample of 10 ml will be collected and stored at −18°C for this purpose. Investigators will remain blinded to these measurements until all patients have completed the randomized part of the study.
Renal artery imaging
INSPiRED will apply state-of-the-art non-invasive imaging of the renal arteries before randomization and during the follow-up. Multi-detector computed tomographic angiography (MDCTA) is the most effective method and will be the technique of choice. MDCTA has the advantage of high spatial and temporal resolution, widespread availability, implantable device compatibility and easy technical reproducibility (Citation28,Citation29). MDCTA can determine the position and orientation of renal arteries, precisely measure the length and diameter of the renal arteries, and visualize lesions and stenosis of the renal arteries. In cases of contra-indications to MDCTA or iodine injection, contrast-enhanced magnetic resonance imaging (MRI) will be used.
Sympathetic modulation
Assessment of heart rate variability is a possible way to investigate changes in autonomous nervous tone after RDN. The high-frequency component of heart rate variability depends on vagal activity, while the low-frequency power predominantly represents sympathetic modulation (Citation30,Citation31). Heart rate variability will be assessed at baseline and at the 6-month follow-up visit, based on 15-min ECG recordings in the supine and standing position according to a published standardized protocol (Citation32,Citation33). To analyse the ECG recordings, a software program, developed by Aubert and colleagues (Citation34) will be used.
Stimulation of the renal nerves before and after ablation allows us to monitor the haemodynamic responses in terms of heart rate and blood pressure. Chinushi and coworkers (Citation35) applied this technique in animals and found that before RDN, electric stimulation was followed by significant increases in blood pressure, heart rate and heart rate variability, as well as circulating epinephrine and norepinephrine, while after the procedure these responses to renal nerve stimulation were significantly blunted at the denervated renal artery, but remained unchanged after application of the same stimuli to the contralateral intact artery. Their study demonstrated that electric stimulation may be a valuable tool to monitor the efficacy of RDN and provide insights into the mechanisms underlying the effects of RDN. During the course of INSPiRED, we will try to develop stimulation of the renal nerves as a novel way to assess completeness of disruption of the renal nerves in selected centres.
Urinary proteomics
Recent publications (Citation36) showed the feasibility of developing multidimensional classifiers based on the urinary proteome that are associated with chronic kidney disease. In the FLEMENGHO population, we observed that the urinary proteome refines the diagnosis of existing or progressing renal dysfunction and predicts cardiovascular complications (unpublished observation). In INSPiRED, investigators will collect spot urine samples at each visit for analysis of the urinary proteome by means of the highly innovative technology of capillary electrophoresis coupled to mass spectrometry (Citation37), developed by Mischak and colleagues (Mosaiques Diagnostics and Therapeutics AG, Hannover, Germany). This technology allows the detection of renal dysfunction at an early subclinical stage.
Morbidity and mortality
Events in INSPiRED will comply with definitions as implemented in previously published randomized clinical trials (Citation38,Citation39).
Quality of life and cost-effectiveness
The quality of life of the patients will be assessed at baseline and at each visit during follow-up, using the EQ-5D questionnaires (http://www.euroqol.org/). Data collected by the EQ-5D questionnaire can be converted into the information provided by the SF-36 instrument. This questionnaire is of particular importance for the economic evaluation and the cost-effectiveness of the RDN. The cost-effectiveness analysis will be conducted from the perspective of the Belgian healthcare system, including both the direct and indirect costs of the intervention. The procedural costs will be balanced against the use of medical resources following RDN, including outpatient visits, hospital admissions, diagnostic procedures, interventions and medications. Adverse events and the incidence fatal and non-fatal events will be recorded for the economic analysis.
Data management and statistical analysis
The SCC in Leuven will be in charge of the management of the trial, the stratification and randomization of patients, database management and statistical analyses. For data management and statistical analysis, the SCC will use SAS software (SAS Institute, Cary, NC, USA). The main analysis will be done using the intention-to-treat dataset. This dataset will include all randomized patients who underwent the procedure and who have at least one endpoint assessed after the randomization. The dataset for observational analyses include complete information on all available patients from screening onwards, irrespective of whether they were randomized. These data will be useful for the analyses attempting to describe the characteristics of patients that will make it to RDN. The per-protocol dataset is a subset of the intention-to-treat dataset excluding patients randomized but not complying with all inclusion and exclusion criteria, patients deviating from the study protocol to such extent that they might introduce bias in the intention-to-treat analysis, and the post-procedural data from patients randomized to the control group, but crossing over to RDN. All statistical tests will be two-sided.
The primary and secondary endpoints measured on a continuous scale will be analysed using mixed models. Comparisons between groups will be done with adjustment for baseline. The fixed effects in the model include randomization group and confounders. To account for the correlation between a patient's repeated blood pressure measurements, the model will also include patient-level random effects. Centre-level random effects might be included to account for the possible correlation of measurements between patients recruited within the same centre. Binary endpoints, such as blood pressure control over time, will be analysed by means of McNemar's test and hierarchical mixed-effects logistic regression models. For endpoints that occur in a given order, the method proposed by Finkelstein & Schoenfeld (Citation40) might be used to combine a time to event and longitudinal event, so that if there is a substantial difference between treatments in either outcome, the null hypothesis will be rejected.
Discussion
INSPiRED is an investigator-steered randomized controlled trial designed to assess the efficacy and safety of RDN. It is powered to address whether RDN on top of usual medical therapy is effective and safe for treating resistant hypertension compared with usual medical therapy alone. The primary and secondary endpoints will be assessed 6 months after randomization, but patients will remain in follow-up up to 36 months.
We decided to launch this new trial, because we strongly believe that previous trials did not select patients most likely to respond to RDN and did not assess efficacy and safety according to the best evidence available from previous studies and current clinical experience in various European countries. The recent announcement that SYMPLICITY HTN-3 failed to reach its primary endpoint of efficacy confirms the need for a radically different approach. Furthermore, as stated in the published analysis plan, failure to meet the primary endpoint of efficacy will preclude analysis of the secondary endpoint based on 24-h systolic blood pressure. The parallel announcement that the primary endpoint for safety (including all-cause mortality, end-stage renal disease and major cardiovascular complications) was achieved is in no way unexpected and of little relevance in the absence of substantial blood pressure lowering efficacy. It does not rule out subtle changes in eGFR, such as those observed in the 3year follow-up of SYMPLICITY HTN-1 patients (Citation41), in whom eGFR fell from 83.6 to 74.3 ml/min/1.73 m2 (p = 0.05). The SYMPLICITY HTN-3 debacle highlights that contrary to the prevailing opinion of the manufacturers, RDN can never replace properly instituted and adjusted treatment with antihypertensive drugs. However, the failure of SYMPLICITY HTN-3 creates new opportunities for well-designed randomized controlled trials of RDN in an attempt to identify the “niche” of patients, who are likely to benefit from the procedure.
INSPiRED is geared to reach its goal because of its unique design features: (i) a stringent selection of patients, excluding patients with chronic kidney disease stage 3 (< 60 ml/min/1.73 m2) and patients with isolated systolic or isolated diastolic hypertension, but including patients with multiple renal arteries with suitable anatomy; (ii) the age range will be limited from 20 to 69 years; (iii) drug optimization with assessment of adherence throughout the study, use of variable single-pill combinations and long acting “forgiving” drugs (Citation20); (iv) 24-h ambulatory blood pressure is applied for patient selection and for assessment of the primary efficacy endpoint; (v) the primary endpoint for safety is based on eGFR estimated according to the CKD-EPI equation; (vi) state-of-the-art renal artery imaging by computerized tomographic angiography with the possibility of using MRI if the former technique is contra-indicated; (vii) validation of urinary proteomic biomarkers to predict blood pressure responses and changes in renal function; (viii) The trial includes an assessment of quality of life and an economical analysis; (ix) extension of the follow- up beyond 6 months up to 3 years to assess the incidence of morbidity and mortality; (x) use of RDN systems with a design different from the SYMPLICITY catheter, and (xi) use of heart rate variability in all patients and renal nerve stimulation in selected centres to assess the completeness of RDN acutely and chronically.
In conclusion, INSPiRED has unique design features that sets this trial apart from all other studies of RDN in treatment-resistant hypertension. INSPiRED will generate long-term efficacy and safety data, identify the subset of treatment-resistant hypertensive patient responsive to RDN, and provide information on cost-effectiveness. The information acquired during the INSPiRED trial will inform guideline committees and health policy makers.
Contributing investigators
AZ Maria Middelares Campus Maria Middelares, Gent – Johan De Sutter, Kristoff Cornelis; AZ Sint Jan Campus Sint-Jan, Brussel – Luc Muyldermans; AZ Turnhout Campus Sint-Elisabeth, Turnhout – John Thoeng; CHR Mons Hainaut, Mons – Michel Henry, Albertino Maimone, Abdelhamid Lalaoui, Philippe Leroy; CHU Dinant Godinne (UCL) site Dinant, Dinant – Gabriela Migali; CHU Dinant Godinne (UCL) site Godinne, Yvoir – Jean- François De Wispelaere, Jean-Michel Pochet, Michel Tintillier. Claude Hanet, Antoine Guedes, Vincent Dangoisse, Laurence Gabriel; Clinique Saint-Luc Bouge, Bouge – Alain Brasseur, Philippe Blouard, Patrick Timmermans; Clinique et Maternité Sainte-Elisabeth (CMSE), Namur – Jean-Michel Pochet, Michel Tintillier, Charles Cuvelier; Grand Hôpital de Charleroi (GHDC), Gilly – Jean-Philippe Lengelé, Marc Carlier, Amandine Jourdan; GasthuisZusters Antwerpen (GZA) ziekenhuizen, Wilrijk – Wouter Vinck, Johan Scharpé; Ziekenhuis Oost-Limburg (ZOL) Hartcentrum, Genk – Mathias Vrolix; Heilig Hart Ziekenhuis Roeselare-Menen (HHRM), Roeselare – Karl Dujardin; Imelda, Bonheiden – Luc Janssens, Bavo Ector; Jessa Ziekenhuis, Hasselt – Philippe Timmermans; Onze-Lieve-Vrouw-Ziekenhuis, Aalst – Marc Goethals; Cliniques Universitaires Saint-Luc (UCL), Bruxelles – Alexandre Persu, Jean-Philippe Lengelé, Jean Renkin, Jean-Benoit le Polain de Waroux, Christophe Scavée, Agnès Pasquet, Frank Hammer, Joëlle Kefer, Francesca Severino, Dominique Huyberechts, Anne Bouvier, Marie Baelen, Erwin Curraj; University Hospitals Leuven, Leuven – Jan A. Staessen, Peter Sinnaeve, Peter Verhamme, Yu Jin, Lotte Jacobs, Lutgarde Thijs, Thibault Petit.
Acknowledgements
The authors gratefully acknowledge the expert assistance of Sandra Covens and Annick de Soete (Studies Coordinating Centre, Leuven, Belgium).
Source of funding
The pilot trial will be funded by own resources made available by the Studies Coordinating Centre in Leuven. The Belgian branch of St. Jude Medical will provide generators and catheters for the pilot study at the two centres (Cliniques Universitaires Saint-Luc, Bruxelles and University Hospitals Leuven).
Conflict of interest
None of the authors declares a conflict of interest.
References
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57: 1076–1080.
- de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Oliveras A, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011;57:889–890.
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197.
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373: 1275–1281.
- Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903–1909.
- Persu A, Renkin J, Thijs L, Staessen JA. Renal denervation – Ultima ratio or standard in treatment- resistant hypertension. Hypertension. 2012;60:596–606.
- Jin Y, Persu A, Staessen JA. Renal denervation in the management of resistant hypertension: Current evidence and perspectives. Curr Opin Nephrol Hypertens. 2013;22: 511–518.
- Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: Insights from meta-analysis of antihypertensive drug trials of 4121 patients with focus on trial design: The CONVERGE report. Heart. 2013;99:1579–1587.
- Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170.
- Staessen JA, Jin Y, Alexandre P, Azizi M, Kjeldsen S. First-in-man randomized clinical trial of renal denervation for atrial arrhythmia raises concern. J Am Coll Cardiol. 2013;62:e445–e446.
- Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008; 371:281–283.
- Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470.
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: Diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419.
- Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009; 2:102–112.
- Kloch-Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, Gonzalez A, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound. 2012;10:10.
- Liu YP, Richart T, Jin Y, Struijker Boudier HA, Staessen JA. Retinal arterial and venular phenotypes in a Flemish population: Reproducibility and correlates. Artery Res. 2011; 5:72–79.
- British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, The Stroke Association. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular diseases in clinical practice. Heart. 2005;91:1–52.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219.
- Hernández-Hernández R, Armas de Hernández MJ, Armas-Padilla MC, Carvajal AR, Guerrero-Pajuelo J. The effect of missing dose of enalapril versus amlodipine on ambulatory blood pressure. Blood Press Monit. 1996;1: 121–126.
- Chobanian AV, Bakris GL, Black BK, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: Results from a randomized trial. Hypertension. 2008;54:475–481.
- O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768.
- O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701.
- Pickering TG, Houston Miller N, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29.
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354.
- Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013; 31:766–774.
- Liu PS, Platt JF. CT angiography of the renal circulation. Radiol Clin North Am. 2010;48:347–365.
- Turba UC, Uflacker R, Bozlar U, Hagspiel KD. Normal renal arterial anatomy assessed by multidetector CT angiography: Are there differences between men and women ?Clin Anat. 2009;22:236–242.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93: 1043–1065.
- Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension. 2002;39:63–68.
- Stolarz K, Staessen JA, Kawecka-Jaszcz K, Brand E, Bianchi G, Kuznetsova T, et al. Genetic variation in CYP11B2 and AT1R influences heart rate variability conditional on sodium excretion. Hypertension. 2004;44: 156–162.
- Stolarz K, Staessen JA, Kuznetsova T, Tikhonoff V, State D, Babeanu S, et al. Host and environmental determinants of heart rate and heart rate variability in four European populations. J Hypertens. 2003;21:525–535.
- Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919.
- Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, et al. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61: 450–456.
- Zürbig P, Jerums G, Hovind P, Macisaac RJ, Mischak H, Nielsen SE, et al. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012;61: 3304–3313.
- Albalat A, Husi H, Stalmach A, Schanstra JP, Mischak H. Classical MALDI-MS versus CE-based ESI-MS proteomic profiling in urine for clinical applications. Bioanalysis. 2014; 6:247–266.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358: 1887–1898.
- Haller H, Ito S, Izzo JL, Jr., Januszewics A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917.
- Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999; 18:1341–1354.
- Krum H, Schlaich MP, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629.
