Abstract
The aim of this study was to investigate the association of serum hyaluronidase and nitric oxide (NO) levels with arterial stiffness in patients with hypertension (HT) and diabetes mellitus (DM). A total of 101 patients with diagnosis of DM and HT were enrolled in this study. The patients were divided into three groups as follows: only hypertensive (I), only diabetic (II) and both diabetic and hypertensive (III). Serum hyaluronidase levels were negatively correlated with aortic strain (AS) and aortic distensibility (AOD) in all groups, whereas a significant positive correlation was noted between serum hyaluronidase levels and aortic strain index (ASI) (all p-values < 0.05). There was a significant negative correlation between serum hyaluronidase and NO levels in all patients (p < 0.001). When the correlation between serum hyaluronidase and serum NO levels was investigated in the individual patient groups, a negative correlation was found in groups I, II and III (p = 0.017, p < 0.001 and p < 0.001, respectively). A significant relationship between plasma hyaluronidase level and parameters of aortic stiffness was found in patients with HT and/or DM. We suggest that the pathophysiological mechanisms responsible for the development of arterial stiffness in subjects with impaired endothelial function may involve pathological changes in the HA metabolism.
Introduction
Arterial stiffness is an early manifestation of adverse structural and functional changes in the vessel wall associated with a wide spectrum of clinical settings including atherosclerosis, hypertension (HT), diabetes mellitus (DM), chronic renal disease, valvular heart disease, hypertrophic cardiomyopathy, congenital heart disease, connective tissue disorders, as well as aging (Citation1). Arterial stiffness is described as the decrease in the compliance of the elastic arterial wall in response to cyclic pressure changes developing in each ventricular ejection. Increased arterial stiffness is known to be related to cardiovascular risk factors such as hypercholesterolemia, HT, DM, metabolic syndrome, as well as obesity, and C-reactive protein levels (Citation2). Mechanical stresses like HT and chemical stresses like inflammation and advanced glycation end-products, which are increased in DM, lead to reparative processes resulting in structural changes in the vessel wall and extracellular matrix. These changes ultimately pave the way to increased atherosclerosis, which by itself is associated with increased collagen deposition and vessel calcification and endothelial dysfunction. These structural changes ultimately lead to increased arterial stiffness, which is clinically reflected as elevations in pulse wave velocity, ventricular workload and systolic blood pressure. The resulting left ventricular hypertrophy, unbalanced myocardial oxygen demand and inadequate coronary perfusion may be associated with myocardial ischemia (Citation1). The endothelium regulates arterial stiffness by release of vasoactive mediators. The presence of endothelial dysfunction as measured with decrease in nitric oxide (NO) mediated vasodilatation is a well-recognized entity among patients with increased arterial stiffness (Citation3).
Hyaluronan (hyaluronic acid, HA) is a glycosaminoglycan involved in water and protein homeostasis of extracellular matrix, cell proliferation and migration (Citation4). HA is an important component of the glycocalyx layer, which is a principal determinant of the vascular permeability barrier by enveloping vascular endothelial cells in a dynamic fashion (Citation5–7). Hyaluronidase, the enzyme involved in the degradation of HA, has several isoforms. Hyaluronidase-1 is unique because it is the only isoform found in the human circulation (Citation8). Alterations in HA metabolism have been previously demonstrated to be related to various vascular abnormalities like, increased vascular permeability to lipoproteins, increased adhesion of mononuclear cells and platelets to the endothelium, and attenuated NO availability (Citation9,Citation10). While the first two mechanisms serve the basis for atherosclerotic changes observed in diabetic patients with coronary artery disease (CAD) in whom plasma HA and hyaluronidase levels were found to be increased (Citation11), the last mechanism may be associated with endothelial dysfunction – a condition known to be related to both arterial stiffness and atherosclerotic vascular disease.
The aim of this study was to investigate the association of serum hyaluronidase and NO levels with arterial stiffness in patients with DM and HT. To the best of our knowledge, this is the first study assessing the relation between serum hyaluronidase levels and arterial stiffness in humans.
Material and methods
A total of 101 patients who had been admitted to the outpatient clinics of a university hospital with diagnosis of DM and HT were enrolled in this study. The patients were divided into three groups as follows: only hypertensive, only diabetic and both diabetic and hypertensive. Each patient underwent a physical examination and clinical information such as age, gender, smoking habits, past medical history including prior medications and chronic illnesses were assessed.
The subjects with systolic blood pressure (SBP) ≥ 140 mmHg, and/or diastolic blood pressure (DBP) ≥ 90 mmHg or current use of antihypertensive medication were classified as hypertensive. DM was defined as fasting glucose levels ≥ 126 mg/dl or hemoglobin (Hb) A1c ≥ 6.5% or being treated with oral antidiabetic drugs or insulin.
Blood pressure was measured with the person in a seated position after a 5-min rest with the Omron HEM 725 CIC electronic, auscultatory blood pressure reading machine (Omron Healthcare Inc., Lake Forest, IL, USA).
Twelve hours fasting venous blood samples were collected by venipuncture using the vacutainer system from Becton Dickinson (Franklin Lakes, NJ) into tubes containing anticoagulant EDTA, centrifuged and obtained sera were stored at − 80°C until used for analysis of hyaluronidase and NO levels. Serum hyaluronidase levels were measured with a commercially available kit using an enzyme-linked immune sorbent assay (ELISA) method (Antibodies-online Inc., Atlanta, GA, USA), and serum NO levels were measured with a commercially available kit (Enzo Life Sciences Inc., NY, USA) using the enzyme immunoassay (EIA) method. Plasma total cholesterol (TC), triglyceride (TG) and high-density lipoprotein-cholesterol (HDL-c) levels were assayed by reagents and standards from the Abbott Diagnostics (Abbott C8000i, Germany). The low-density lipoprotein-cholesterol (LDL-c) was assayed by applying the Friedwald's Formula for samples with TG ≤ 400 mg/dl (Citation12). The height and weight were recorded and body mass index (BMI) was calculated as the ratio of weight in kilograms divided by the square of height in meters.
Aortic elasticity parameters were measured just before blood sampling by an experienced cardiologist blinded to the patients’ clinical features, using a 2.5–3.5-mHz probe on a Vingmed System V echocardiography machine (GE-Vingmed Ultrasound AS, Horten, Norway). Ascending aorta was evaluated in the parasternal long-axis view using M-mode internal diameters obtained 3 cm above the aortic valve. Diastolic aortic diameter (AoD) was measured at the tip of the R wave in the simultaneous ECG recording and systolic aortic diameter (AoS) was measured at the maximal anterior motion of the anterior aortic wall. Blood pressure was measured in each patient using a properly sized cuff sphygmomanometer and systolic (SBP) and diastolic (DBP) pressures were recorded along with their heart rates. Aortic elasticity indices were calculated as follows: aortic strain (AS) = 100×(AoS− AoD)/AoD; aortic strain index (ASI) = (SBP/DBP)/AS; aortic distensibility (AOD) = 2 × AS/pulse pressure, where pulse pressure is SBP− DBP (Citation3).
Patients with following characteristics were excluded: usage of concomitant drugs capable of changing NO levels (e.g. non-steroidal anti-inflammatory drugs, dexamethasone, phosphodiesterase type 5 inhibitors, nitroglycerine, isosorbide dinitrate and mononitrate, sodium nitroprusside), chronic alcohol intake, history of peripheral or coronary artery disease, heart failure, valvular heart disease, signs of any systemic inflammatory disease, history of recent infections, concomitant renal or hepatic disease, cancer, history of recent major surgery or trauma, sub-optimal echocardiographic trans-thoracic image quality, and pregnancy.
Written informed consent was obtained from all the participants and the study was approved by the local ethics committee and institutional review board. The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
The values were expressed as mean± standard deviation (SD). Variables were analyzed for presence of normal distribution using Kolmogorov–Smirnov and Shapiro–Wilk tests. Differences of continuous and categorical variables between three groups were compared using the analysis of variance (ANOVA) test. Correlations between variables were assessed using Pearson's test. A p-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software version 14.0 (SPSS for Windows, Version 14.0, SPSS Inc., Chicago, IL, USA).
Results
A total of 101 patients (35 males and 66 females; mean age 56.77 ± 6.78 years) were included in the present study. There were 21 patients with only type II DM (group I: five men and 16 women), 30 patients with only HT (group II: 14 men and 16 women), and the remaining 50 patients (group III: 16 men and 34 women) had both DM and HT. Baseline demographic, clinical and biochemical characteristics of the patients are given in . When the groups were compared with regard to age, gender, smoking habits, diastolic blood pressure, BMI and lipid profiles, no significant differences were found (all p-values > 0.05).
Table I. Baseline demographic, clinical and biochemical characteristics of patients.
The data regarding the drugs that the study patients were given at the time of the study were as follows: angiotensin-converting enzyme inhibitors (n = 44), angiotensin receptor blockers (n = 32), calcium channel blocker (n = 38), beta-blocker (n = 26), alpha-blocker (n = 16), diuretics (n = 48), oral antidiabetics (n = 58) and insulin (n = 22). All hypertensive patients were receiving antihypertensive medication at the time of the study. Likewise all diabetic patients were on either oral antidiabetic drugs and/or insulin at the time of the study.
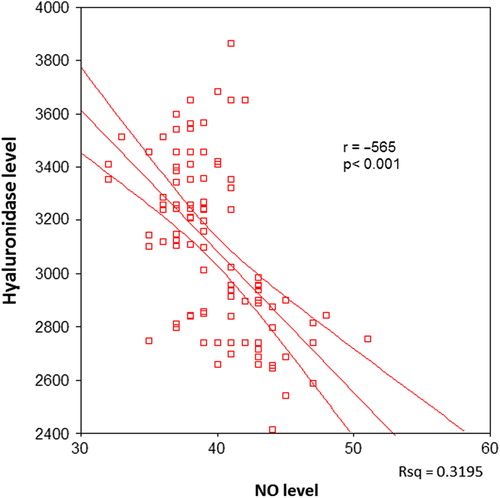
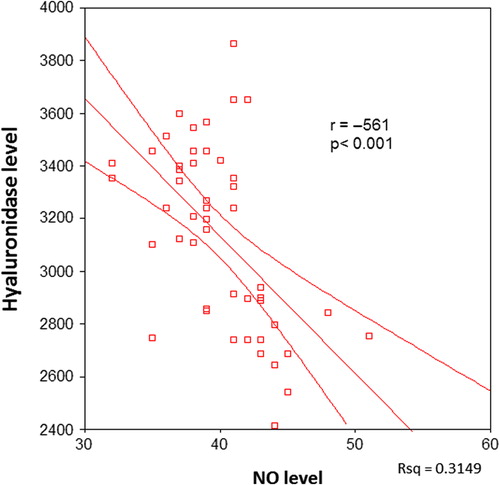
There was a significant negative correlation between serum hyaluronidase and NO levels in all patients (p < 0.001, ). When the correlation between serum hyaluronidase and serum NO levels was investigated in the individual patient groups, a negative correlation was found in groups I, II and III (p = 0.017, p < 0.001 and p < 0.001, respectively) ().
Figure 2. The correlation between serum hyaluronidase and nitric oxide (NO) levels in only diabetic patients.
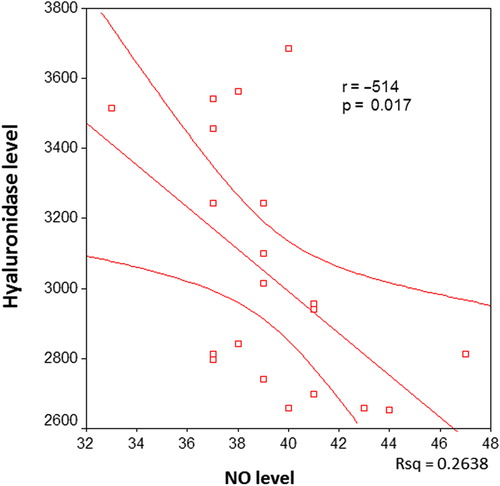
Figure 3. The correlation between serum hyaluronidase and nitric oxide (NO) levels in hypertensive patients.
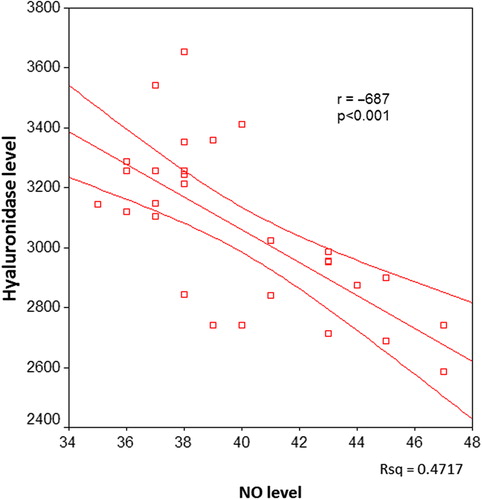
Figure 4. The correlation between serum hyaluronidase and nitric oxide (NO) levels in hypertensive and diabetic patients.
The relation of serum hyaluronidase levels with parameters of aortic elasticity including AS, ASI and AOD was also evaluated in the present study. In groups I, II and III, a significant negative correlation was found between serum hyaluronidase levels and AS and AOD, whereas a significant positive correlation was noted between serum hyaluronidase levels and ASI (all p-values < 0.05, ). In groups I, and III, a significant positive correlation was found between serum NO levels and AS and AOD (all p-values < 0.05, ), whereas in group II, the correlation between serum NO levels AS and AOD and was insignificant (p = 0.096 and p = 0.25, respectively). A significant negative correlation was noted between serum NO levels and ASI in groups I, II and III (all p-values < 0.05, ).
Table II. The relation of serum hyaluronidase and nitric oxide levels with parameters of aortic elasticity including aortic strain, aortic strain index and aortic distensibility.
Discussion
The main findings of the present study were as follows: (i) a significant relationship between plasma hyaluronidase level and parameters of aortic stiffness was found in patients with HT and/or DM. There was an inverse association between aortic elasticity and serum hyaluronidase levels. (ii) A significant negative correlation was demonstrated in all patients between hyaluronidase levels and NO, which is an important indicator of endothelial function. (iii) NO levels were found to be negatively correlated with aortic stiffness in all patient groups as demonstrated by one but not all the measured aortic elasticity parameters.
Structural and functional changes of the arterial wall, which take place due to aging, environmental and genetic factors, lead to decreased elasticity of the arterial wall and hence increased arterial stiffness (Citation13–15). The correlation between arterial stiffness and cardiovascular adverse events like myocardial infarction and stroke is a recognized clinical entity. Arterial stiffness increases with age and it is known to be directly related to cardiovascular risk factors like HT, DM, metabolic syndrome, hyperlipidemia, obesity and elevated C-reactive protein levels (Citation1). Structural changes that take place in the arterial wall during the stiffening process include changes in the amount and density of the predominant scaffolding proteins like collagen and elastin. On the other hand, recent immunohistochemical and ultrastructural studies suggest that the spatial organization of the stiff wall material also affects arterial stiffness (Citation16). HA is an extracellular matrix component, which is known to be altered in terms of its distribution and amount in the formation of atherosclerotic plaques (Citation17–19). The removal of glycocalyx layer with hyaluronidase is shown to be associated with increased vascular permeability leading to atherosclerosis (Citation20,Citation21). HA has been suggested to influence arterial smooth muscle cell proliferation and migration during the development of atherosclerosis (Citation18,Citation22,Citation23). HA may also serve as a substrate for migration of inflammatory cells as monocytes and lymphocytes into the atherosclerotic lesion as a part of an inflammatory reaction (Citation24). Increased plasma HA and hyaluronidase levels have been found to be associated with endothelial glycocalyx damage, presence of microvascular disease and atherosclerosis in diabetics (Citation11,Citation25). We have demonstrated, for the first time, a significant relation between serum hyaluronidase levels and aortic stiffness parameters in patients with HT and/or DM.
Hyaluronidase, the enzyme responsible for the degradation of HA, has been demonstrated to decrease the shear stress-related NO production in a canine model (Citation2). The degradation of HA glycosaminoglycans within the glycocalyx layer was demonstrated to decrease flow-induced NO production indicating that HA is an essential element for detection and amplification of flow induced shear force toward the cell membrane, and thus endothelial function. It has also been suggested that in high-oxidative stress states such as HT and DM, the decreased NO production may be related to effects of shear stress stimuli being less than normal (Citation26). In the present study, we were able to demonstrate a significant negative correlation between hyaluronidase levels and NO in patients with HT and/or DM. Endothelial dysfunction has been previously reported to be associated with arterial stiffness in healthy subjects, hypertensive patients and diabetics (Citation27–29). We were able to demonstrate that NO levels were found to be negatively correlated with aortic stiffness in hypertensive subjects, diabetics and diabetic patients with HT. Hyaluronidase levels have been demonstrated to be increased in patients with coronary artery disease (Citation30). Although we have not enrolled patients with clinically apparent atherosclerotic vascular disease, we cannot rule out the contribution of atherosclerosis to hyaluronidase levels in our patient population given the possibility of clinically silent atherosclerosis in diabetics and hypertensives.
Study limitations
The study is limited due to the relatively small number of patients and its cross-sectional design. The changes in HA metabolism during the time course of HT and DM and the relationship between HA and progression of aortic stiffness remain to be determined. Ambulatory blood pressure measurement data, if they were available, would estimate the blood pressure control status of the hypertensive subjects more reliably. Moreover, the lack of target organ damage assessment by using conventional imaging modalities such as echocardiography and carotid ultrasound may be considered other limitations of this study. The clinical value of measurement of indices of HA metabolism requires further studies to be elucidated.
Conclusion
We suggest that the pathophysiological mechanisms responsible for the development of arterial stiffness in subjects with impaired endothelial function may involve pathological changes in the HA metabolism. Further studies are needed to specify the exact role of HA in the development of aortic stiffness related to endothelial dysfunction in HT and DM.
Acknowledgments
This study was not financially supported. The authors have no relevant conflicts of interest to disclose.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605.
- Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217.
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277: 4593–4596.
- Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486.
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514.
- van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594.
- Frost GI, Csóka AB, Wong T, Stern R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem Biophys Res Commun. 1997;236:10–15.
- Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr Opin Lipidol. 2005;16:507–511.
- Kornerup K, Nordestgaard BG, Feldt-Rasmussen B, Borch-Johnsen K, Jensen KS, Jensen JS. Increased transvascular low density lipoprotein transport in insulin dependent diabetes: A mechanistic model for development of atherosclerosis. Atherosclerosis. 2003;170:163–168.
- Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55: 1127–1232.
- Tremblay AJ, Morrissette H, Gagné J.-M, Bergeron J, Gagné C, Couture P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with b-quantification in a large population. Clinical Biochemistry. 2004;37:785–790.
- Nichols WW, O’Rourke MF. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles. 3rd ed. London: Edward Arnold; 1990. p 77–142, 216–269, 283–359, 398–437.
- Safar ME. Pulse pressure in essential hypertension: Clinical and therapeutical implications. J Hypertens. 1989;7:769–776.
- Laurent S, Kingwell B, Bank A, Weber M, Struijker-Boudier H. Clinical applications of arterial stiffness: Therapeutics and pharmacology. Am J Hypertens. 2002;5:453–458.
- Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055.
- Toole BP, Wight TN, Tammi MI. Hyaluronan–cell interactions in cancer and vascular disease. J Biol Chem. 2002; 277:4593–4596.
- Papakonstantinou E, Roth M, Block LH, Mirtsou-Fidani V, Argiriadis P, Karakiulakis G. The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration. Atherosclerosis. 1998;138:79–89.
- Lévesque H, Girard N, Maingonnat C, Delpech A, Chauzy C, Tayot J, et al. Localization and solubilization of hyaluronan and of the hyaluronan-binding protein hyaluronectin in human normal and arteriosclerotic arterial walls. Atherosclerosis. 1994;105:51–62.
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514.
- van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594.
- Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996; 75:249–262.
- Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147.
- Ferns GA, Konneh M, Rutherford C, Woolaghan E, Anggard EE. Hyaluronan (HYAL-BV 5200) inhibits neo-intimal macrophage influx after balloon-catheter induced injury in the cholesterol-fed rabbit. Atherosclerosis. 1995; 114:157–164.
- Nieuwdorp M, Holleman F, de Groot E, Vink H, Gort J, Kontush A, et al. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia. 2007;50:1288–1293.
- Mochizuki S, Dahlöf B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Jikei Heart Study group. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): A randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007; 369:1431–1439.
- Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro Moraes C, et al. Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press. 2012;21:31–38.
- McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48: 602–608.
- Shan Y, Lin J, Xu P, Zeng M, Lin H, Yan H. The combined effect of hypertension and type 2 diabetes mellitus on aortic stiffness and endothelial dysfunction: An integrated study with high-resolution MRI. Magn Reson Imaging. 2014;32: 211–216.
- Kucur M, Karadag B, Isman FK, Ataev Y, Duman D, Karadag N, et al. Plasma hyaluronidase activity as an indicator of atherosclerosis in patients with coronary artery disease. Bratisl Lek Listy. 2009;110:21–26.