Abstract
Background. Increased levels of inflammatory biomarkers such as interleukin-6 (IL-6), 10 (IL-10), 1β (IL-1β), tumor necrosis factor-α (TNF-α) high-sensitivity C-reactive protein (hs-CRP) are associated with arterial stiffness in hypertension. Indeed, resistant hypertension (RHTN) leads to unfavorable prognosis attributed to poor blood pressure (BP) control and target organ damage. This study evaluated the potential impact of inflammatory biomarkers on arterial stiffness in RHTN. Methods. In this cross-sectional study, 32 RHTN, 20 mild hypertensive (HTN) and 20 normotensive (NT) patients were subjected to office BP and arterial stiffness measurements assessed by pulse wave velocity (PWV). Inflammatory biomarkers were measured in plasma samples. Results. PWV was increased in RHTN compared with HTN and NT (p < 0.05). TNF-α levels were significantly higher in RHTN and HTN than NT patients. No differences in IL-6 levels were observed. RHTN patients had a higher frequency of subjects with increased levels of IL-10 and IL-1β compared with HTN and NT patients. Finally, IL-1β was independently associated with PWV (p < 0.001; R2 = 0.5; β = 0.077). Conclusion. RHTN subjects have higher levels of inflammatory cytokines (TNF-α, IL-1β and IL-10) as well as increased arterial stiffness, and detectable IL-1β levels are associated arterial stiffness. These findings suggest that inflammation plays a possible role in the pathophysiology of RHTN.
Introduction
Resistant hypertension (RHTN) is defined as blood pressure (BP) that remains above goals despite of the concurrent use of three antihypertensive agents of different classes, including a diuretic, at optimal dose amounts. Also, patients whose BP are controlled but require four or more medications are considered resistant (Citation1). Patients were evaluated for adherence to treatment (Citation2) and underwent antihypertensive therapy optimization. Increased BP leads to organ damage via hemodynamic load, and presumably, RHTN patients have unfavorable prognosis attributed to extended time of poor BP control and other associated cardiovascular risk factors (Citation3,Citation4).
Risk stratification in patients with arterial hypertension depends not only on BP levels but also on additional cardiovascular risk factors as well as identification of target organ damage (TOD) (Citation5). Arterial stiffness is a strong and independent predictor of all-cause and cardiovascular mortality in hypertensive patients (HTN) (Citation6). The relationship between high BP levels and arterial stiffness may be explained, at least in part, by inflammatory pathways (Citation7). In addition, it has been demonstrated that higher BP stimulates an inflammatory response through mechanical and hormonal stimuli (Citation8).
Inflammatory biomarkers such as interleukin 6 (IL-6), interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) have been associated with hypertension and TOD (Citation7,Citation9,Citation10). Furthermore, levels of IL-6, TNF-α and high-sensitivity C-reactive protein (hs-CRP) were positively correlated with pulse wave velocity (PWV) in HTN patients (Citation7). Moreover, hs-CRP levels were associated with left ventricular hypertrophy in RHTN subjects (Citation11). On the other hand, IL-10, an anti-inflammatory cytokine blunted vascular inflammation induced by angiotensin II in mice (Citation12). Taken together, these findings pointed out the role of these inflammatory markers on pathophysiology of hypertension.
In fact, some studies have demonstrated antihypertensive drugs such as angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blockers (ARBs) and mineralocorticoid receptor (MR) antagonist could exert beneficial cardiovascular effects due to anti-inflammatory effects (Citation13–15).
This study compared levels of inflammatory biomarkers (IL-6, IL-10, TNF-α and IL-1β) and arterial stiffness among RHTN patients, mild to moderate hypertension (HTN) subjects and normotensive. Also, we investigated the potential impact of inflammatory cytokines (IL-6, TNF-α and IL-1β) on arterial stiffness.
Methods
Patient population
A cross-sectional study was performed in the Outpatient Resistant Hypertension Clinic at the University of Campinas Hospital. Thirty-two patients classified as RHTN and 20 mild to moderate (stages I and II) hypertension (HTN) patients were recruited. In addition, 20 normotensive volunteers matched for age, gender and body mass index (BMI) were enrolled as control group. The diagnosis of RHTN required a proper office BP measurement technique and ambulatory BP monitoring (ABPM) to confirm persistently elevated BP levels. All patients were followed and treated for a period of at least 6 months with regular scheduled appointments before characterized as resistant to treatment. We performed physical examination, complete medical history inquiry, electrocardiogram and laboratory testing in all individuals. Exclusion criteria included secondary hypertension (identifiable and removable causes of hypertension, including Conn's or Cushing's syndrome, diabetes, renal artery stenosis, pheochromocytoma, coarctation of the aorta), liver and renal disease, heart failure (ejection fraction < 50%), stroke, peripheral vascular disease, smokers, obesity (BMI ≥ 30 kg/m²), pregnancy or oral contraceptive use, history or clinical evidence of recent infection and use of anti-inflammatory drugs.
HTN patients were evaluated for adherence to treatment (Citation2) and underwent antihypertensive therapy optimization. Adherence was measured by pill count each time the patients returned for their scheduled appointments. Patients with pill counts ≥ 80% were classified as adherent (Citation16). Adherence was also assessed using Morisky's questionnaire (Citation17), a four-“yes-or-no”-question test that evaluates attitudes regarding medication intake. Only patients who scored 4 points were considered highly adherent to treatment. Also, non-pharmacological therapies were optimized, including salt and caloric restriction. The patients gave the written informed consent form before enrolling in the study approved by Research Ethics Committee at the Faculty of Medical Sciences, University of Campinas, São Paulo, Brazil (approval no. 222/2011) and the procedures were performed in accordance with the Declaration of Helsinki.
Blood pressure measurements
Brachial BP from each patient (SBP and DBP) was measured twice in the right arm by a trained healthcare professional, with the patient in the seated position and using a validated digital sphygmomanometer (HEM-907XL, OMRON Healthcare Inc., Bannockburn, IL, USA.). These measurements were assessed according to the AHA Scientific Statement (Citation1).
The 24-hour ABPM was evaluated using a Spacelabs 90217 ambulatory BP monitor (Spacelabs Inc, Redmon, WA, USA). Patients were instructed to maintain normal daily activities and to note their sleep period in a personal diary.
Laboratory assessments
Blood samples were collected at 8:00 h after overnight fasting. The plasma levels of cytokines IL-1β, IL-6, IL-10 and TNF-α were determined by enzyme-linked immunosorbent assay (ELISA) in duplicates (R&D Systems, Inc., Minneapolis, USA) according to manufacturer's instructions. In addition, serum cholesterol, low-density lipoprotein-cholesterol (LDL), high-density lipoprotein-cholesterol (HDL), triglycerides, glucose, aldosterone, creatinine and hs-CRP were measured.
PWV measurement
PWV is the “gold standard” method to evaluate arterial stiffness. This method requires little technical expertise and PWV has been employed as a marker of vascular damage in patients with cardiovascular risk factors. PWV was measured using the SphygmoCor System (Atcor Medical, Sydney, Australia) with the patient in the supine position. Pulse waves of the carotid and femoral arteries were analyzed, estimating the delay with respect to the ECG wave. Distance measurements were taken with a measuring tape from the sternal notch to the carotid–femoral recording site. Carotid–femoral PWV was calculated by dividing traveled distance by transit time (PWV = distance/time). We performed at least two measurements. In the case of differences higher than 0.5 m/s, a third measurement was performed. The PWV value was reported as the mean, whose values were corrected for mean arterial pressure. A logarithmic transformation for PWV values was used to achieve normal distribution and perform multiple linear regression.
Statistical analysis
Continuous variables were expressed as means, standard deviations (SD) and 95% confidence intervals (CI). The Kruskal–Wallis test was used to compare three groups with pos hoc analysis done by Dunn's multiple comparison test. The Mann– Whitney test was used to compare two groups. IL-1β and IL-10 were categorized according to minimum detectable concentration, thus being < 0.012 or > 0.012 pg/ml and < 1.0 or > 1.0 pg/ml respectively. Categorical data were presented in percentages and compared by chi-square test. The impact of inflammatory biomarkers (IL-10, TNF-α, IL-1β and hs-CRP) on the PWV was assessed through multiple linear regression analysis adjusted for age, office SBP and glucose. The level of significance (α) accepted was less than 0.05.
Results
General patient characteristics are shown in . No statistical differences were observed among the groups regarding age, gender and body mass index (BMI). As expected, highest office SBP and pulse pressure (PP) were found in RHTN patients compared with HTN and NT subjects, but not in DBP measurements (). In addition, systolic ABPM was higher in RHTN subjects compared with HTN patients. Biochemical parameters showed no differences among the three groups, except for glucose levels and hs-CRP, which were higher in both hypertensive groups compared with control ().
Table I. General characteristics of normotensive, mild to moderate hypertensive and resistant hypertensive subjects.
Table II. Biochemical parameters of normotensive, mild to moderate hypertensive and resistant hypertensive subjects.
RHTN patients were taking a mean of 4.3 ± 0.9 antihypertensive drugs while HTN subjects 2.3 ± 0.6 (p < 0.001). The drugs distribution in percentage in RHTN group was 100% diuretics, 34% spironolactone, 44% ACE inhibitors, 44% ARBs, 84% calcium channel blocker (CCB), 84% beta-blockers, 31% central nervous system-acting antihypertensive drugs and 13% others. HTN subjects received 80% diuretics, 5% spironolactone, 40% ACE inhibitors, 30% ARBs, 30% CCB and 45% beta-blockers. The frequency of statins use in RHTN and HTN groups were 34% and 35%, respectively.
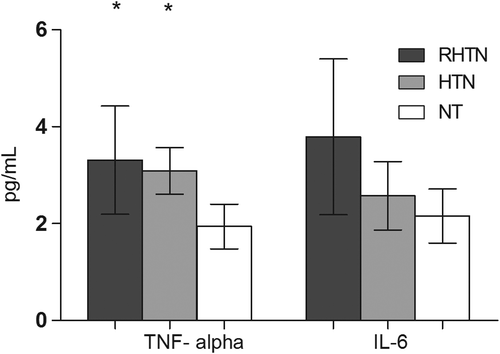
We found a higher proportion of subjects in RHTN group with elevated IL-10 levels (> 1.0 pg/ml) compared with HTN and NT (78% vs 50% and 20%) groups, p = 0.0002. Similarly, RHTN patients showed higher frequency of IL-1β levels > 0.012 pg/ml compared with HTN and NT (100% vs 45% and 25%, respectively, p < 0.0001) (). Mean TNF-α levels were significantly higher in RHTN and HTN subjects (3.3 and 3.1 pg/ml, respectively) compared with NT individuals (1.94 pg/ml), p = 0.0018 (). However, no differences were observed between the groups with respect to IL-6 levels ().
Figure 1. Frequency of patients according interleukin 1β (IL-1β) and interleukin 10 (IL-10) levels in normotensive, mild to moderate hypertensive and resistant hypertensive subjects (chi-square test: p = 0.0002 and p < 0.0001, respectively). NT, normotensive; HTN, mild to moderate hypertension; RHTN, resistant hypertension.
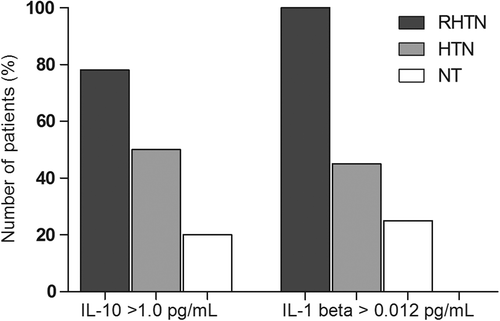
Figure 2. Comparison between tumor necrosis factor- α (TNF-α) and interleukin 6 (IL-6) levels in normotensive, mild to moderate hypertensive and resistant hypertensive subjects. (mean (95% CI). NT, normotensive; HTN, mild to moderate hypertension; RHTN, resistant hypertension). p = 0.0018. *vs NT.
PWV was higher in RHTN (10.5 ± 2.2 m/s) compared with HTN (8.7 ± 2.1 m/s) patients and both groups had higher values compared with NT individuals (7.2 ± 1.0 m/s), p < 0.0001 (). Multiple logistic regression analysis revealed that IL-1β, but not IL-10, TNF-α and hs-CRP, is predictor of PWV in all groups (p < 0.001; R2 = 0.50; β = 0.077) ().
Figure 3. Arterial stiffness (pulse wave velocity, PWV) in normotensive (NT), mild to moderate hypertensive (HTN) and resistant hypertensive subjects (RHTN). p < 0.0001 *vs NT; #vs HTN.
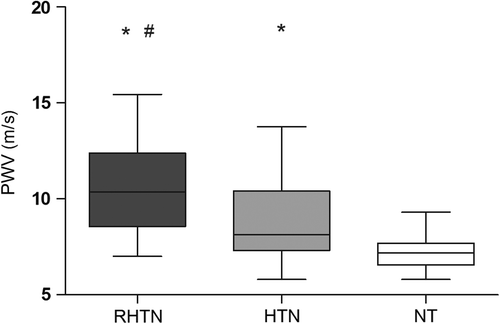
Table III. Multivariate linear regression analysis for the associations between inflammatory biomarkers [interleukin 1β (IL-1β), interleukin 10 (IL-10), tumor necrosis factor-α (TNF-α) and high-sensitivity C-reactive protein (hs-CRP)], and arterial stiffness of normotensive, mild to moderate hypertensive and resistant hypertensive subjects.
Discussion
This study evaluated the relationship between circulating levels of inflammatory cytokines on arterial stiffness in RHTN patients. We showed higher arterial stiffness (determined by PWV) in the RHTN group compared with HTN and NT groups, and IL-1β levels were independently associated with arterial stiffness. Also, our findings pointed out that IL-1β and IL-10 levels were higher in RHTN patients compared with mild to moderate HTN patients and NT subjects, despite of the use of multiple antihypertensive drugs in the former group. TNF-α levels were higher in both hypertensive groups compared with NT, but no differences were found between RHTN and HTN patients.
Arterial stiffness is a recognized risk factor for heart disease that can precede and thus contribute to hypertension. It occurs as a consequence of structural changes in connective tissue proteins within vascular wall (Citation18). PWV is the “gold standard” method to evaluate arterial stiffness and strong evidence has demonstrated its predictive value for cardiovascular outcomes (Citation19). Our research group previously demonstrated a close relationship between high BP levels, increased PWV and endothelial dysfunction in RHTN patients when compared with well-controlled HTN patients (Citation20). This association suggests a possible role of arterial stiffness in the pathogenesis of RHTN. Previous studies highlighted that arterial stiffness and RHTN share some associated conditions such as obesity, aging, diabetes, chronic renal disease as well as isolated systolic hypertension (Citation21,Citation22). We found that despite general characteristics were similar among the three studied groups, RHTN subjects have increased arterial stiffness, suggesting other mechanisms may be associated with arterial stiffness in RHTN patients. These findings strengthen the importance to investigate other factors that can be involved in arterial stiffness in RHTN patients such as vascular inflammation.
Inflammatory responses contribute in both structural and functional changes in the arterial wall, and have been emerged as a potential determinant of arterial stiffness (Citation7,Citation8,Citation23). In fact, a positive correlation between IL-6, TNF-α and hs-CRP with PWV in HTN has already demonstrated in essential hypertension (Citation7). Also, hs-CRP, which is used in clinical practice for risk stratification in cardiovascular diseases (Citation24), was associated with target organ damage in RHTN subjects (Citation11,Citation25). Evidence showed that lowering inflammation, demonstrated through hs-CRP levels, may decrease vascular events’ rates (Citation26). However, this is the first study to investigate the relationship between inflammatory cytokines and arterial stiffness in RHTN patients. Our study demonstrated that hypertensive subjects (RHTN and HTN groups) had higher hs-CRP and RHTN subjects also had increased levels of the cytokines IL-10, IL-1β and TNF-α. In addition, we found that IL-1β is independently associated with arterial stiffness.
The link between inflammatory cytokines and hypertension has been showed in animal models and in human studies (Citation10,Citation12,Citation27). Previous studies reported that TNF-α plasma levels are associated with elevated BP in apparently healthy subjects (Citation9,Citation28). In fact, hypertensive subjects had higher levels of TNF-α compared with normotensive volunteers. Supporting the role of this inflammatory cytokine in hypertension, we previously demonstrated that neutralization of circulating TNF-α reduces BP in spontaneously hypertensive rats (Citation29). The renin–angiotensin–aldosterone system (RAAS) plays an important role in regulation of vascular tone. It is well known that Ang II is one of the major mediators of vascular remodeling in hypertension, and has pro-inflammatory properties by inducing the production of cytokines, including TNF-α, IL-6 and IL-1β (Citation14,Citation30) We found that the RHTN group had significantly higher number of subjects with elevated IL-1β levels compared with HTN and NT groups. Similarly, a longitudinal study found that higher levels of IL-1β and interleukin 1 receptor antagonist (IL1-ra) are associated with increases in systolic BP and the development of hypertension in normotensive subjects (Citation10). It was previously demonstrated that the infusion of IL-1β in rats induced vasopressor responses leading to BP elevation (Citation27). In our study, the independent association of IL-1β and PWV, even after adjustment for age and SBP, reflects the importance of this inflammatory marker in arterial stiffness development. In addition, a large prospective long-term study demonstrated that IL-1ra levels are strong predictors of aortic stiffness (Citation31). Further supporting the cause-and-effect relationship between inflammatory pathways in artery stiffness process, an experimental study demonstrated that acute systemic inflammation increases arterial stiffness in healthy individuals (Citation32). Taken together, these findings provide evidence that pro-inflammation may precede or even contribute to BP elevation and arterial stiffness.
Furthermore, another component of the RAAS, aldosterone, causes vascular injury by inducing oxidative stress and inflammation by activate mineralocorticoid receptor (MR) (Citation33). Evidence in humans showed that Ang II or aldosterone infusion increases plasma IL-6 concentrations and that blockade of MR attenuates IL-6 raises during Ang II infusion (Citation14). We found no difference in IL-6 levels among groups and it might be explained by interference due to the higher proportion of the RHTN patients were using spironolactone, a mineralocorticoid receptor antagonist. Also, an imbalance among IL-6, TNF-α and IL-1β levels is biologically plausible. Increases in IL-6 levels, after an induced systemic inflammatory response in healthy volunteers, were followed by unchanged levels of IL-1β and TNF-α (Citation34). Indeed, it has been suggested that IL-6 may mediate the synthesis and release of IL-1 and TNF antagonists, counter-regulating TNF-α and IL-1β levels (Citation35).
On the other hand, RHTN patients showed highest levels of an anti-inflammatory cytokine, IL-10, followed by HTN and NT subjects. The anti-inflammatory properties of IL-10 have been proposed as a systemic immune response to counteract Ang II-induced inflammation (Citation12). This is supported by the observation that IL-10 levels increase with the infusion of Ang II. Also, Ang II–induced hypertension was prevented and IL-10 levels were normalized after adoptive transfer of T regulatory cells in mice lacking T cells (Citation12). However, it was demonstrated that Ang II infusion leads to endothelial dysfunction in IL-10 knockout animals, but not in controls (Citation36). An inverse association of IL-10 with diastolic BP, endothelial dysfunction was reported in obese hypertensive subjects (Citation37). Our findings can be interpreted as a counter-regulation mechanism in response to the vascular inflammation in RHTN patients.
Antihypertensive agents, including ACE inhibitors and ARB antagonists or their combination with statins could exert beneficial anti-inflammatory effects (Citation13,Citation15). Nevertheless, in the present study, RHTN subjects, even taking several antihypertensive drugs, had higher levels of IL-1β and IL-10 compared with HTN patients, suggesting that the increased level of inflammation was not been counteracted by medication, which may be contribute to the increased cardiovascular risk in RHTN patients. In addition, our findings showed no differences between the RHTN and HTN groups with respect to TNF-α levels, but both groups had higher TNF-α levels compared with apparently healthy volunteers. Our findings suggest that these inflammatory biomarkers may be related to the degree of hypertension.
The disruption in inflammatory cytokines levels might be related to target organ damage and cardiovascular risk in RHTN patients and may be a possible therapeutic target to this condition. The importance of identifying those at risk for cardiovascular disease in order to prevent new events is emerging.
The limitations of the present study are the small number of patients enrolled and the lack of standard in the use of antihypertensive drugs, due to individualized care. Defining true treatment RHTN has been highly debated (Citation38). In order to select only true RHTN patients, who were on optimal doses and confirmed adherence, the low prevalence of true RHTN is observed (Citation38). In the present study, adherence was measured by pill count and questionnaire. Also, we cannot exclude the interference of drugs taken by the patients in the inflammatory cytokines analyzed. However, the withdrawn of such medications is not feasible due to ethical concerns. Despite this limitation, RHTN patients, even on multiple antihypertensive agents, had elevated cytokine levels and higher arterial stiffness.
In summary, our study demonstrates that RHTN patients, despite of multiple antihypertensive therapy have increased cytokines levels compared with mild to moderate HTN and NT subjects. In addition, higher levels of IL-1β are associated with increased arterial stiffness and may contribute to increased cardiovascular risk. The findings of this current study need to be confirmed in prospective clinical studies using larger RHTN population.
Notice of correction
The label on the y-axis of Figure 3 was updated post publication online.
Conflict of interest: The authors have no conflicts of interest to declare.
This study was supported by the State of São Paulo Research Foundation (FAPESP), SP, Brazil, National Council for Scientific and Technological Development (CNPq) and Coordination for Improvement of Higher Education Personnel (Capes), Brazil.
References
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419.
- de Souza WA, Yugar-Toledo JC, Bergsten-Mendes G, Sabha M and Moreno H, Jr. Effect of pharmaceutical care on blood pressure control and health-related quality of life in patients with resistant hypertension. Am J Health Syst Pharm. 2007;64:1955–1961.
- Martins LC, Figueiredo VN, Quinaglia T, Boer-Martins L, Yugar-Toledo JC, Martin JF, et al. Characteristics of resistant hypertension: Ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens. 2011;25:532–538.
- Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, et al. Refractory hypertension: Definition, prevalence, and patient characteristics. J Clin Hypertens (Greenwich). 2012;14:7–12.
- Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertens. 2009; 27:2121–2158.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Mahmud A and Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005; 46:1118–1122.
- Chappell DC, Varner SE, Nerem RM, Medford RM and Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539.
- Bautista LE, Vera LM, Arenas IA and Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154.
- Mauno V, Hannu K and Esko K. Proinflammation and hypertension: A population-based study. Mediators Inflamm. 2008;2008:619704.
- Salles GF, Fiszman R, Cardoso CR and Muxfeldt ES. Relation of left ventricular hypertrophy with systemic inflammation and endothelial damage in resistant hypertension. Hypertension. 2007;50:723–728.
- Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476.
- Brili S, Tousoulis D, Antoniades C, Vasiliadou C, Karali M, Papageorgiou N, et al. Effects of ramipril on endothelial function and the expression of proinflammatory cytokines and adhesion molecules in young normotensive subjects with successfully repaired coarctation of aorta: A randomized cross-over study. J Am Coll Cardiol. 2008;51:742–749.
- Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48:1050–1057.
- Takagi H, Mizuno Y, Yamamoto H, Goto SN and Umemoto T. Effects of telmisartan therapy on interleukin-6 and tumor necrosis factor-alpha levels: A meta-analysis of randomized controlled trials. Hypertens Res. 2012;36:368–373.
- Sackett DL, Haynes RB, Gibson ES, Hackett BC, Taylor DW, Roberts RS, et al. Randomised clinical trial of strategies for improving medication compliance in primary hypertension. Lancet. 1975;1:1205–1207.
- Morisky DE, Green LW and Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74.
- Duprez DA and Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007; 9:139–144.
- Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid–femoral pulse wave velocity. J Hypertens. 2012;30:445–448.
- Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro Moraes C, et al. Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press. 2012;21:31–38.
- Pabuccu T, Baris N, Ozpelit E, Akdeniz B and Guneri S. The relationship between resistant hypertension and arterial stiffness. Clin Exp Hypertens. 2012;34:57–62.
- Protogerou A, Blacher J, Stergiou GS, Achimastos A and Safar ME. Blood pressure response under chronic antihypertensive drug therapy: The role of aortic stiffness in the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind) study. J Am Coll Cardiol. 2009;53:445–451.
- Pietri P, Vyssoulis G, Vlachopoulos C, Zervoudaki A, Gialernios T, Aznaouridis K, et al. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24:2231–2238.
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367: 1310–1320.
- Magen E, Mishal J, Paskin J, Glick Z, Yosefy C, Kidon M, et al. Resistant arterial hypertension is associated with higher blood levels of complement C3 and C-reactive protein. J Clin Hypertens (Greenwich). 2008;10:677–683.
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207.
- Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T and Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens. 1992;5:224–229.
- Ito H, Ohshima A, Tsuzuki M, Ohto N, Takao K, Hijii C, et al. Association of serum tumour necrosis factor-alpha with serum low-density lipoprotein-cholesterol and blood pressure in apparently healthy Japanese women. Clin Exp Pharmacol Physiol. 2001;28:188–192.
- Filho AG, Kinote A, Pereira DJ, Renno A, dos Santos RC, Ferreira-Melo SE, et al. Infliximab prevents increased systolic blood pressure and upregulates the AKT/eNOS pathway in the aorta of spontaneously hypertensive rats. Eur J Pharmacol. 2013;700:201–209.
- Rosa AC, Rattazzi L, Miglio G, Collino M and Fantozzi R. Angiotensin II induces tumor necrosis factor-alpha expression and release from cultured human podocytes. Inflamm Res. 2012;61:311–317.
- Johansen NB, Vistisen D, Brunner EJ, Tabak AG, Shipley MJ, Wilkinson IB, et al. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS One. 2012; 7:e37165.
- Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200.
- Briet M and Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2012;50:89–99.
- Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999.
- Tilg H, Trehu E, Atkins MB, Dinarello CA and Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83: 113–118.
- Didion SP, Kinzenbaw DA, Schrader LI, Chu Y and Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54: 619–624.
- Fonseca HA, Fonseca FA, Monteiro AM, Bianco HT, Boschcov P, Brandao SA, et al. Obesity modulates the immune response to oxidized LDL in hypertensive patients. Cell Biochem Biophys. 2013.
- Moreno H, Jr., Coca A. Resistant and refractory hypertension: Reflections on pathophysiology and terminology. Blood Press. 2012;21:209–210.
