Abstract
Background. Treatment of resistant hypertension has attained much attention during the past few years and naturally so has the prevalence of resistant hypertension. In the search for sources of such documentation, the lack of blood pressure (BP) control in randomized clinical outcome trials in hypertension has been used as indication of treatment-resistant hypertension. In the present study, we aimed at using previously unpublished information from monitoring of clinical trials in investigating the mechanism explaining why large fractions of patients in the trials remained uncontrolled for their high BP. Methods. We report insight information from LIFE (n = 9193), VALUE (n = 15,245), ASCOT (n = 19,257) and ACCOMPLISH (n = 11,506). Data stored during the course of the trials for monitoring purposes were scrutinized for fractions of patients with BP control, which was BP < 140/90 mmHg in all trials, and we identified monitoring data showing fractions of patients who had been uptitrated to the various dosing levels or combinations of study drugs in the trials. Fractions of patients who had not been uptitrated on drugs and who remained without BP control identified the level of physician (investigator) inertia in these trials. Results. In the LIFE Study the majority of patients remained with systolic BP > 140 mmHg throughout. Approximately 1500 patients remained on the first dose titration step despite not having reached target BP. In the VALUE Trial 59.5% had reached systolic BP target 2 years into the study; 23.9% of patients remained on the lowest study dose and only 15.1% had been uptitrated to the highest study dose. In the ASCOT Trial, as many as 28% of participants had not reached target diastolic BP at year 4 in the study, and of these patients 37% still remained on the first drug dose titration step. In the ACCOMPLISH Trial approximately 80% had achieved the systolic BP target at study end; however, during the course of the trial approximately 25% of participants remained uncontrolled and at 6 months almost 60% of these patients had not been titrated to the highest drug dose level. Conclusion. These data, taken from the monitoring phases of large outcome trials in hypertension, show that inertia, the lack of titration of study drugs to higher dosing levels or drug combinations according to the study protocols, is a major cause of not reaching BP targets in the trials. Thus, fractions of patients not reaching BP targets in outcome trials cannot be taken as evidence of treatment-resistant hypertension.
Introduction
Treatment of resistant hypertension is currently attracting huge attention and naturally so is the search for the prevalence of resistant hypertension. In the large Spanish database (Citation1) containing 60,045 patients, 8295 patients (12.2%) were considered treatment resistant according to common criteria with treatment with three drugs in maximal doses including a diuretic, but only 62.5% of these again, a total of about 8%, remained treatment resistant after assessment with 24-h ambulatory blood pressure (BP). In the USA, recent data suggest that large fractions of uncontrolled hypertensive patients are merely undertreated (Citation2). As new treatment methods have great potentials, it is important to clarify the true prevalence of treatment-resistant hypertension in the population and particularly in hypertension databases and studies. In the search for sources of such documentation, the lack of BP control in randomized clinical outcome trials in hypertension has been put forward as verification of treatment-resistant hypertension. In the present study, we aimed at using previously unpublished information from monitoring of clinical trials in investigating the potential mechanisms why large fractions of patients in the trials remained uncontrolled for their high BP.
Methods
We have insight information from the Losartan Intervention For Endpoint reduction in hypertension study (LIFE) (n = 9193), the VAlsartan Longterm Use Evaluation Trial (VALUE) (n = 15,245), the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) (n = 19,257) and the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension study (ACCOMPLISH) (n = 11,506) (Citation3–6). Some previous publications have reported in detail the levels of BP control during the course of the trials (Citation7–9). For the purpose of this report, the data stored with one of the investigators (SEK) during the course of the trials for monitoring purposes were scrutinized for fractions of patients with BP control, which was systolic BP < 140 and diastolic BP < 90 mmHg in all trials, and we identified monitoring data showing fractions of patients who had been uptitrated to the various dosing levels or combinations of study drugs in the trials. Fractions of patients who had not been uptitrated on drugs and who remained without BP control identified the level of physician (investigator) inertia in these trials. The original databases for monitoring during the blinded phases of the trials, resting with the sponsors or study statisticians, have not been accessed. These databases would in all cases bee different from the cleaned and locked databases that have been used to analyse and report main outcome data (Citation3–6). We present graphs and tables that were originally used for the monitoring purposes, and presented at investigators’ meetings during the course of the trials. Quite extensive series of power point slides with such data from various time points throughout the trials have been collected and stored, but for the sake of clarity and making the main points, only a few but representative graphs or tables have been selected and included in this paper. Data are given as number of patients (n) or fractions of patients in % reaching certain BP goals or being titrated to certain dose levels or drug combinations. It is not possible to merge the data sets, not even approximations, because monitoring tools and presentation set-ups were different between trials, and thus specific statistical analyses on these data have not been performed.
Results
The Losartan Intervention For Endpoint reduction in hypertension study (LIFE)
The average systolic BP remained > 150 mmHg after 12 months in the study () and the majority of patients remained with systolic BP > 140 mmHg throughout. Approximately 1500 patients remained on the first dose titration step of the blinded study drug (50 mg of losartan or atenolol) despite not having reached target systolic BP, and they had thus not been uptitrated according to the study protocol by the investigators. The fractions of patients exposed to such inertia were similar in all seven participating countries (in relation to patients included in the various countries; , ).
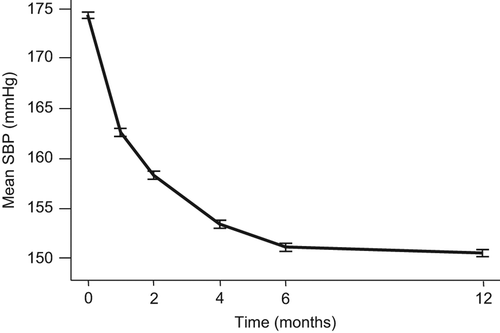
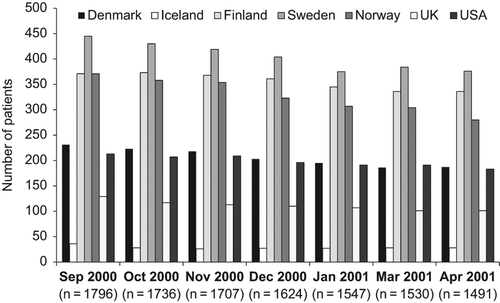
Table I. Number of patients per country with systolic blood pressure > 140 mmHg and remaining on the initial dose (50 mg) of test drug (not uptitrated by investigator) from September 2000 to April 2001 in the LIFE study.
The VAlsartan Longterm Use Evaluation Trial (VALUE)
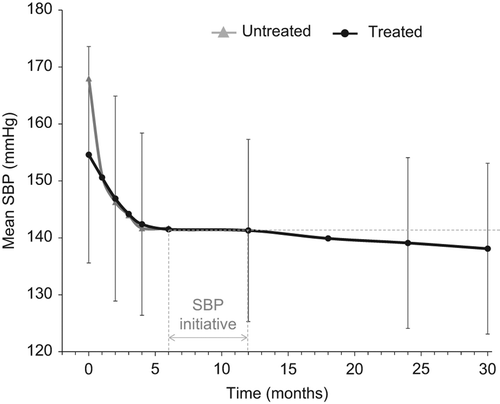
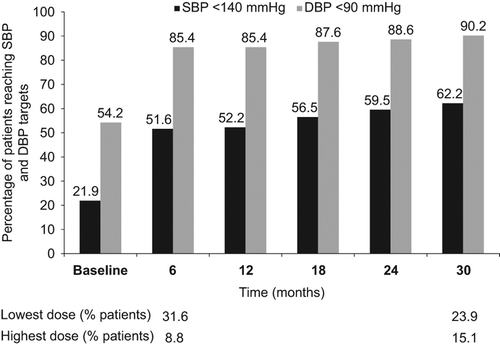
The average systolic BP was 141 ± 16 mmHg after 12 months of treatment in the Value trial and the Steering Committee launched an initiative to improve BP control (). In the VALUE Trial, 59.5% had reached systolic BP target 2 years into the study; at this time 23.9% of patients remained on the lowest study dose of the blinded medication according to the study protocol (5 mg of amlodipine or 80 mg of valsartan) and only 15.1% had been uptitrated to the highest study dose (10 mg of amlodipine or 160 mg of valsartan combined with 25 mg of hydrochlorothiazide) ().
The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT)
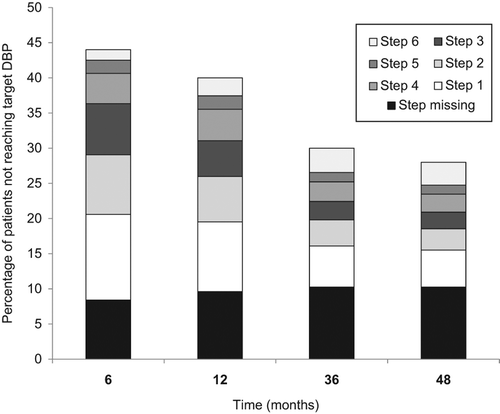
As many as 44%, 40%, 30% and 28% of ASCOT participants had not reached target diastolic BP at 6 months and 1, 3 and 4 years into the study (). Within this fraction of participants who had not reached target diastolic BP, 19%, 24%, 34% and 37% of the patients, respectively, still remained on the first dose drug titration step (5 mg amlodipine or 1.25–2.5 mg of bendroflumethiazide) and a corresponding 28%, 25%, 19% and 19% remained on the second dose titration step at these time points in the study ().
The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension study (ACCOMPLISH)
In ACCOMPLISH, approximately 80% had achieved the systolic BP target at study end (, ); however, during the course of the trial approximately 25% of participants remained uncontrolled and at 6 months almost 60% of these patients had not been titrated to the highest dose level of medication, which was a free add on in addition to the maximal doses of blinded study drug combinations (). For the diabetic subpopulation, a target BP < 130/80 mmHg was recommended, and among the 56% who had not reached this level of BP control at 6 months, almost 65% had not been titrated to the highest drug dose level with free add on, as recommended by the protocol ().
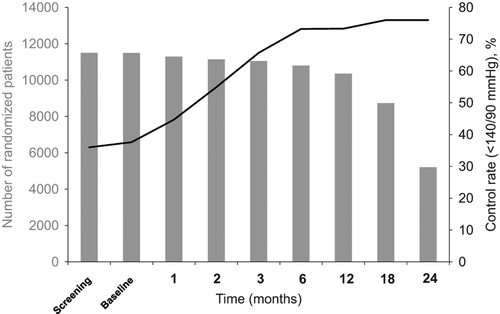
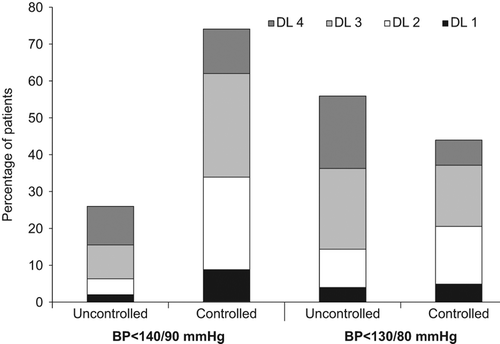
Table II. Blood pressure control (< 140/90 mmHg) for all randomized patients in the ACCOMPLISH trial (as of October 2006).
Discussion
Fractions of patients not reaching target BP < 140 mmHg, < 90 mmHg or combination < 140/90 mmHg ranged from above 50% in the LIFE study to less than 50% in the ASCOT Trial, and approximately 40% in the VALUE Trial and 20% in the ACCOMPLISH study. ACCOMPLISH thus achieved the best BP control ever seen in any large prospective randomized outcome trial in hypertension (Citation6). However, irrespective of intermediate or excellent BP control, our data taken from the monitoring phases of the trials show that the lack of titration of study drugs to higher dosing levels or drug combinations according to the study protocols is a major cause of not reaching BP targets in these trials.
We use the lack of titration of study drugs to higher doses or combination treatment according to the protocols as a proxy of inertia in these trials. Clearly, fractions of patients not reaching BP targets in outcome trials cannot be taken as evidence of treatment-resistant hypertension. We cannot rule out that there are sub-fractions of patients who would eventually show treatment resistance if they had been properly titrated to more medication according to the protocols. Such analyses can be performed; not on the fractions of patients not being uptitrated to at least three drugs in full doses being discussed in this paper, but rather by identifying patients in the entire study populations given full doses of at least three drugs and still not reaching BP targets. This has apparently been done for ASCOT and about half of the entire study population was considered to have treatment-resistant hypertension (Citation10). However, home or ambulatory BPs were not investigated and many uncertainties applied (Citation11). It appears that the analysis assumed that “Per-protocol, the antihypertensive therapy of every patient was up-titrated at every follow-up visit according to a predefined BP-management algorithm, if BP targets had not been reached”, thus not taking lack of up-titration by investigators into account and grossly overestimating the number of treatment-resistant patients by mixing “uncontrolled” with “resistant” hypertension. ASCOT and ACCOMPLISH contained study arms with 50% of patients not given diuretics, unless as a free add on in a limited number of patients, further making it difficult to assess the true number of treatment-resistant hypertension inasmuch as the definition includes ensuring that patients take a diuretic in full or the highest tolerated dose. In LIFE and VALUE, this could be more appropriately done with diuretic (hydrochlorothiazide) given up to 25 mg, which is considered a full dose, but ambulatory BPs were only taken on limited subgroups only.
Treatment inertia is per definition an untoward exercise by physicians who are supposed to prescribe drugs as needed and follow clinical guidelines or other recommendations. The phenomenon is well know and has been described before, e.g. in a study of 2595 patients investigated in Spain, where treatment modifications were not performed in 75% of 13792 visits to the physicians’ offices despite BP not being controlled (Citation12). In the present study, the situation is slightly different in as much as patients participated in randomized clinical outcome trials; thus the term investigator inertia seems more appropriate than physician inertia. Anyway, a medical doctor or physician is responsible for sites also in the clinical trials and study technicians or nurses perform work delegated by the medical doctor. Another aspect is the countries participating, which have been of major importance in LIFE and ASCOT in particular, as these studies contained the majorities of patients in Scandinavian countries. Here, general practitioners known for their rather conservative approach towards drug treatment of hypertension were mostly responsible for patients’ treatment and study conduct. However, ACCOMPLISH also contained a sizable fraction of Scandinavian patients and was able to overcome this problem to a much larger extend, probably due to the forced titration aspect of the protocol (Citation9).
A critical question remains: why was there a high rate of physician non-compliance with the protocol requirements in these trials, particularly as most investigators were regarded as experts in treating hypertension? There are at least four possible additional explanations:
| (1) | Some patients were obtaining additional antihypertensive drugs that were not authorized by the study protocols and thus were not reported. For instance, investigators might have added beta-blockers in patients with cardiac conditions or angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients whose renal function was deteriorating or who had developed diabetes. | ||||
| (2) | Investigators might have known or suspected that some patients were poorly compliant with their study drugs (a phenomenon of “therapeutic fatigue” that can occur in long-term clinical trials) and so believed that adding further drugs would be futile. | ||||
| (3) | Not all experts agree that a systolic BP target of < 140 mmHg is always justified, even if a trial protocol calls for it. This might be the case in older patients where there can be concern over excessive reductions in diastolic BP. | ||||
| (4) | Some investigators are concerned about large numbers of drugs in their patients, especially those needing therapy for conditions other than hypertension. So, if a patient's BP falls to within a few mmHg of the target, they might decide not to add a further drug. | ||||
Beyond these practical issues, another explanation for poor BP control in these trials is the artificiality of the treatment regimens often required by the study protocols. Most experts now believe – unless there are compelling reasons to do otherwise – that the ideal three-drug regimen for BP control is a renin–angiotensin system blocker plus a calcium channel blocker plus a thiazide diuretic. However, to protect the integrity of their hypothesis testing, most of the major trials (including LIFE, VALUE, ASCOT and ACCOMPLISH) did not allow this optimal therapy in most or all of their patients. However, irrespective of the internal study and monitoring issues discussed in this paper of up-titration of study drugs and investigator inertia, LIFE, VALUE, ASCOT and ACCCOMPLISH have been so far the randomized outcome trials comparing different drugs or drug combinations in essential hypertension that had statistical power to investigate properly the hypotheses that they aimed to investigate (Citation13). Other trials have been underpowered (Citation14) or investigated other populations with established disease and not necessarily hypertension (Citation15), but in none of them should uncontrolled hypertension be misinterpreted as treatment-resistant hypertension (Citation16).
The publication of the data in this paper is somewhat limited by the lack of access to the original databases, which were used during the monitoring phases of the trials. We were thus not able to merge the data from the four trials. Furthermore, as discussed thoroughly above, we cannot provide distinct explanations why patients were not up-titrated according to study protocol. Such mechanisms deserve detailed investigations in future prospective and randomized outcomes trials in hypertension.
In summary, these data, taken from the monitoring phases of large outcome trials in hypertension, show that the lack of titration of study drugs to higher dosing levels or drug combinations according to the study protocols, a proxy of physician inertia, is a major cause for not reaching BP targets in the trials. Thus, fractions of patients not reaching BP targets in outcome trials cannot be taken as evidence of treatment-resistant hypertension. Additionally, our data show that investigator inertia must be seriously considered when designing large outcome trials in hypertension in the future.
Disclosures: SEK, SJ, BD and MAW report no relevant conflicts of interest to disclose related to this article. The main contents of this report were included in Lennart Hansson Memorial Lecture at ESH Annual Scientific Meeting.
References
- De la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cuz JJ, Armariao P et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011; 57:898–902.
- Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to2008. Circ.2011;124: 1046–1058.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U et al. for the LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Julius S, Kjeldsen SE, Weber M, Brunner H, Ekman S, Hansson L et al. Cardiac events, stroke and mortality in high-risk hypertensives treated with valsartan or amlodipine: Main outcomes of The VALUE Trial. Lancet. 2004;363:2022–31.
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers G, Caulfield M et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomized controlled trial. Lancet. 2005; 366:895–906.
- Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V et al. for the ACCOMPLISH trial investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008; 359:2417–28.
- Kjeldsen SE, Dahlöf B, Devereux RB, Julius S, Faire U, Fyhrquist F et al. for the LIFE Study Group. Lowering of blood pressure and predictors of response in patients with left ventricular hypertrophy. Am J Hypertens. 2000;13:899–906.
- Julius S, Kjeldsen SE, Brunner H, Hansson L, Platt F, Ekman S et al. for the Value Trial. VALUE Trial: Long-term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hypertens. 2003; 16:544–8.
- Jamerson K, Bakris GL, Dahlöf B, Pitt B, Valesquez E, Gupte J et al. for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: The ACCOMPLISH trial. Blood Press. 2007, 16:80–86.
- Gupta AK, Nasothimiou EG, Chang CL, Sever PS, Dahlöf B, Poulter NR on behalf of the ASCOT investigators. Baseline predictors of resistant hypertension in the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT): A risk score to identify those at high-risk. J Hypertens. 2011;29: 2004–13.
- de Leeuw PW. Resistance to antihypertensive treatment: Reality or artifact of reasoning? J Hypertens. 2011;29:1861–2.
- Redón J, Coca A, Lázaro P, Aguilar MD, Cabañas M, Gil N, Sánchez-Zamorano MA, Aranda P. Factors associated with therapeutic inertia in hypertension: Validation of a predictive model. J Hypertens. 2010;28:1770–7.
- Schultz M, Aksnes TA, Høieggen A, Kjeldsen SE. Choice of drug in hypertension. Tidsskr Nor Laegeforen. 2013;133: 1802–3. doi: 10.4045/tidsskr.13.0311.
- McInnes GT, Kjeldsen SE. Never mind the quality, feel the width – ALLHAT revisited. Blood Press. 2004;13:330–4.
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New Engl J Med. 2000;342:145–153.
- Modolo R, Faria APD, Sabbatini AR, Moreno H. Resistant hypertension revisited: Definition and true prevalence. J Hypertens. 2014;32:1546.