The Lancet has been instrumental in publishing studies on renal sympathetic denervation (RDN) in hypertension and, accordingly, has strongly contributed to the promotion of this technology to be applied in patients with apparent treatment-resistant hypertension (Citation1–3). However, with the recent publication of the Symplicity HTN-3 study in the USA (Citation4), the world has seemingly overnight become in doubt whether RDN lowers blood pressure at all. An Editor of another distinguished journal (Citation5) published his reflections and stated that the Symplicity HTN-3 results came as a shock to the world; a single but large and properly designed prospective randomized clinical trial could on its own neutralize hundreds of mostly observational studies, case reports and other enthusiastic publications emphasizing the amazing effects of RDN, not only in patients with resistant hypertension, but also in a host of other diseases and conditions.
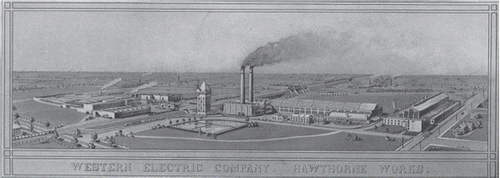
The initial enthusiasm followed by the setback of RDN can probably be summarized by a handful of reflections and explanations: (i) the role of the sympathetic system in the pathophysiology of hypertension is substantiated by a wealth of experimental and clinical arguments (Citation6–12). On this background, enthusiasm surged when an intervention in this system seemed to drastically lower blood pressure. (ii) Market-driven industry took control and exerted an unprecedented influence on the medical community. (iii) Subsequently, pitfalls in apparent treatment- resistant hypertensive patients, which are simple but have been well known for decades, were suddenly forgotten including well described phenomena such as the placebo effect, regression to the mean, poor drug adherence (Citation13–15) and the Hawthorne effect (http://en.wikipedia.org/wiki/Hawthorne_Works).
The history of the rise and fall of RDN – a perceived miracle in the treatment for millions of hypertensive patients – deserves a more in-depth analysis. The first and for a long time the only prospective randomized clinical trial in this field, the Symplicity HTN-2 study (Citation2) was monitored by Ardian (Medtronic), who collected and processed the data (Citation2). Usually, when such a task is given to industry, all measures are taken to secure confidence and show that the trials are prospective, randomized and double-blinded (Citation16–18). However, in this case, everything was open, making the trial particularly vulnerable to patients, physicians and sponsor-related biases (Citation19). Shun-Shin et al. (Citation20) in a recent editorial nicely stated that “measurement of a noisy variable by un-blinded optimistic staff is a known recipe for calamitous exaggeration”. It is unfortunate that the selection of patients enrolled in Symplicity HTN-2 and the evaluation of efficacy were based on office rather than ambulatory blood pressure measurement (ABPM), which is state-of-the art (Citation21), particularly in resistant hypertension (Citation22). ABPM reduces observer bias and measurement error, minimizes the white-coat effect and has greater reproducibility, and therefore provides a better estimate of a patient's usual blood pressure and cardiovascular prognosis (Citation23,Citation24). Notwithstanding the well-known, major contribution of poor drug adherence to apparently resistant hypertension (Citation13–15), drug adherence was not monitored, either at baseline or during follow- up. This made the study vulnerable to the Hawthorne effect (http://en.wikipedia.org/wiki/Hawthorne_Works, see ), i.e. patients changing behaviour – in this case starting taking their drugs as prescribed – in response to the intervention and massive attention devoted to them. The lack of blood pressure decrease in the control group also raises concerns. One would indeed suspect that patients in the control group had not taken their medications properly, in order to keep their blood pressure at a higher level that made them eligible to cross over to the RDN group (Citation25,Citation26). Finally, the placebo effect and regression to the mean must also be taken into account. Simply by recruiting patients with the highest blood pressures at the outset would yield more blood pressure decrease at follow-up. It is noteworthy that the placebo effect is small using ABPM (Citation21,Citation27); however, ambulatory blood pressures remain as sensitive to the Hawthorne effect as office blood pressure.
Figure 1. Hawthorne was an electrical power plant in Chicago (http://en.wikipedia.org/wiki/Hawthorne_Works). Some investigators discovered in the 1920s that the employees worked much harder if lights were turned brighter. But the workers had been informed of the experiment and it was after a while understood that they did not respond to the lights being improved, but simply changed their behaviour because they were under observation. In no other field of medicine is this phenomenon more powerful than in hypertension research. Simply teaching people to take their own blood pressure (Citation49) is changing their behaviour and improving their drug adherence. It is thus no surprise that apparent treatment-resistant hypertensive people who have a mixed motivation for taking their drugs, to a certain degree explaining why they appear as drug-resistant, start taking their drugs following renal sympathetic denervation (RDN), with a subsequent dramatic, but largely non-specific blood pressure fall.
Despite the major limitations and potential biases of Symplicity HTN-2, a small open study with a suboptimal design including only 106 patients followed up for 6 months, RDN was adopted in hundreds of centres worldwide. Medtronic Inc.® (Minneapolis, Minnesota) paid $800 million to purchase Ardian® (Mountain View, California), the company that had developed the technology (Citation5), and more than 10 companies developed their own RDN systems, five of which obtained the CE mark. The procedure was quickly adopted in Germany, and later in Switzerland, Sweden and the Netherlands. While RDN remained an investigational procedure in the USA, at least 8000 (Citation28), possibly 15,000–20,000 procedures were performed in Europe and in the rest of the world in less than 4 years, most of them using the Ardian–Medtronic® catheter. It may be hypothesized that the massive incomes generated by selling the Symplicity catheter to enthusiastic Europeans contributed to the expenses of the Symplicity HTN-3 study (Citation4), required by the Food and Drug Administration before approval of RDN in the USA. In Symplicity HTN-3 (Citation4), blinding of patients through the use of a sham procedure and wider use of ABPM balanced and limited the differential impact of the Hawthorne, white coat, placebo and regression to the mean effects in both treatment arms, disclosing to the world the true size of the blood pressure decrease attributable to RDN, at least in patients meeting the Symplicity criteria; it was less than 2 mmHg systolic based on ABPM.
For all the aforementioned reasons, and in view of the complexity and multifactorial character of hypertension, the failure of RDN to normalize or substantially reduce blood pressure in all patients with apparently resistant hypertension was a reasonable working hypothesis for us, even before the Medtronic announcement that Symplicity HTN-3 had failed to meet its primary endpoint (http://www.tctmd.com/show.aspx?id = 123265). We (Citation29–31) and others (Citation19,Citation25) had predicted that the true effect of RDN might have been overestimated and may considerably shrink in properly designed studies (Citation19), and that “one size may not fit all” (Citation26). In particular, in preliminary analysis of the European Network COordinating research on Renal Denervation (ENCOReD) network (Citation32), we were struck by the imbalance between the 17.6 mmHg decreases in office blood pressure, vs. only 5.9 mmHg for 24-h ambulatory blood pressure.
When we set out to investigate the effects of RDN in one of the centres with the longest experience in conducting randomized clinical trials in Europe (Citation33), we had thus clearly in mind the limitations of previous studies. We needed a simple and practical way to deal with pitfalls in the recruitment of patients with resistant hypertension into a study protocol. After extensively ruling out secondary hypertension, and improving drug treatment as well as possible bias based on clinical judgements in the run-in phase, patients had to qualify for the RDN protocols by having elevated daytime ambulatory blood pressures after witnessed intake of their prescribed blood pressure medication (Citation30). This was a convenient way to identify the true treatment-resistant hypertensive patients and to exclude patients with white coat hypertension or those non-adherent patients whose blood pressure normalized after witnessed drug intake. Meanwhile, a leading hypertension centre in Germany (Citation34) published a small but well documented series of patients whose blood pressure remained unchanged after RDN. For many hypertension experts in Europe, this was a clear sign of what was to come. We were thus not surprised when we found no change in either office or ambulatory blood pressures following RDN, first in an open series of six patients (Citation30), later followed by a randomized study – the Oslo-RDN trial (Citation35). Patients who were randomly assigned to further improvement of drug treatment guided by non-invasive haemodynamic monitoring had normalized blood pressures. In contrast, patients exposed to RDN experienced only a small and probably partly placebo-induced fall in office and ambulatory blood pressures (). The decreases averaged 20 mmHg more for office and 9 mmHg more for ambulatory systolic blood pressure in the haemodynamically guided drug treatment group compared with the RDN group.
Table I. Characteristics and results of three prospective and randomized studies of blood pressure lowering effects of renal sympathetic denervation (RDN) with Symplicity catheters.
In the absence of solid evidence of efficacy, how can we explain the uncontrolled deployment of RDN in Europe and worldwide (with the notable exception of the USA where RDN remained an investigational procedure)? Of course, publications of the Symplicity studies and of multiple observational studies, and enthusiastic editorials and reviews in The Lancet (Citation1–3) and other top-ranking journals such as Circulation (Citation36) and the Journal of the American College of Cardiology (Citation37) had a substantial impact, and the lack of strict rules for introduction of device-based therapies in Europe facilitated the large-scale implementation of the technique. However, this phenomenon would have remained limited without huge promotion by device-producing industry. Probably never before has industry launched a stronger campaign to market a new technology. A multitude of national and international advisory boards organized educational meetings, developed a website (www.poweroverpressure.com) and produced guidelines. Medical journals were swamped by reviews and meta-analyses showing the powerful blood pressure lowering effects as recorded in observational studies and in the single available randomized study, Symplicity HTN-2. Comments pointing out the defects and inconsistencies in such meta-analysis encountered a great delay in being published (Citation38). Physicians were invited to training sessions and the sponsor promoted RDN in large as well as in small hospitals, public and private clinics and facilitated with all means the recruitment of patients to physicians and centres who would perform the procedure. Many never questioned whether RDN should be implemented, but when it should start in an institution. The purpose was to disseminate the enthusiasm for RDN from the technically oriented invasive radiologists and cardiologists who usually had little interest or experience in the treatment of hypertension to the “hypertension establishment”. The European Society of Hypertension issued specific guidelines (Citation39,Citation40), but maintained reservations that more data was needed, and eventually it had to be proved that RDN would lower morbidity and mortality before being generally accepted in the treatment of true or apparent treatment-resistant hypertension.
Unfortunately, the most enthusiastic proponents of RDN do not seem to have fully accepted the lessons of Symplicity HTN-3. In the aftermath of Symplicity HTN-3, a campaign has been set up to criticize the study because of including inexperienced investigators, and enrolling too many African American patients (Citation28,Citation41). It has been suggested that the lack of demonstrated efficacy of RDN in Symplicity HTN-3 may be due to lack of statistical power or even to chance (Citation28), or that the trial was well conceived but not rigorously executed (Citation41). Furthermore, the Symplicity HTN-3 results are diluted by non-scientific comparisons with the Medtronic® registry (Citation42), which is hampered by all the weaknesses touched upon in this commentary, and even more as it is a pure industry-ran activity. Finally, while RDN will not become available in the USA, and ongoing research in Asia was stopped, Medtronic and other companies continued making their catheters available for clinical use in Europe and did not restrain from heavily promoting the technique, for example at the Euro PCR conference in Paris in May 2014 (www.medscape.com/author/shelley-wood).
Does the failure of Symplicity HTN-3 mean the end of RDN? Not necessarily. Indeed, as already mentioned, RDN is based on a solid rationale substantiated by over 50 years of meticulous research of the sympathetic nervous system and its involvement in the pathophysiology of hypertension (Citation6–12). Furthermore, it has been shown in cohorts recruited from the 1930s (The effect of progressive sympathectomy on blood pressure, Walter Bradford Cannon 1931, http://www.ncbi.nlm.nih.gov/pubmed/2204236) until the 1950s (Citation43,Citation44) that abdominal sympathectomy associated to splanchnicectomy is effective in the treatment of severe hypertension. Finally, many centres report major responses to RDN in a minority of patients (Citation30,Citation32,Citation35). Accordingly, research should go on to determine the minority of patients who are true responders to RDN, and identify predictors of effective RDN (). The European Network for Coordinating Renal Denervation (ENCOReD) has been set up to include thousands of patients in randomized protocols, observational studies and registries independent of industry. Some early results (Citation32,Citation45) from this joint effort have already been published and suggest that it may be worthwhile searching for potential predictors of response to RDN.
Figure 2. Oscillations in the development of renal denervation. Courtesy of Peter Sever (Imperial College, London) and published in the April 2014 newsletter of the International Society of Hypertension (http://ish-world.com/news/a/April-issue-of-Hypertension-News/). Based on a cartoon of the uptake of new drugs by Desmond Lawrence.
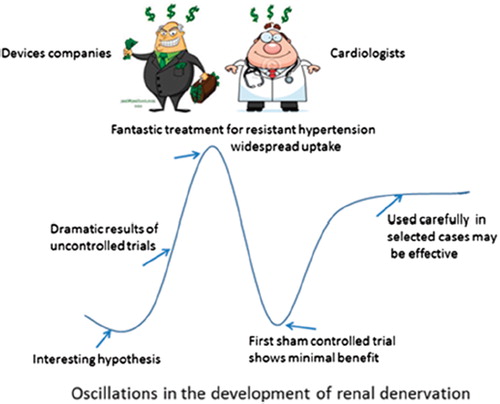
Still, before going ahead, we have to draw the lessons of the RDN story. The wisdom hereof is firstly that no new bright idea can suddenly appear and resolve the problem of hypertension – or even resistant hypertension – as a whole. Previous knowledge has been building up through clever research by generations of investigators and hypertension cannot be resolved overnight. Hypertension is too complex and multifactorial, and the size of the problem so extensive that nobody in this field should let things like the commercial marketing of RDN happen in the way it did. We must make sure that RDN is beneficial and does no harm. Many patients have probably undergone unneeded procedures. By a careful estimate, 20 000 renal arteries have been exposed to ablation in people with hypertension and an increasing number of cases of renal artery stenosis after RDN are reported (Citation46). Very recent news along these lines is that in Germany the insurance companies have terminated their coverage. It remains to be seen whether the negative news that RDN is not for most people will reach Time Magazine (Citation47) and Der Spiegel (Citation48).
Declaration of interests: Sverre E. Kjeldsen has received lecture and consultancy honoraria from AZ, Bayer, Medtronic, MSD, Novartis, Serodus, and Takeda, unrestricted grants from AZ, Hemo Saphiens and Pronova and royalty payments from Gyldendal. Fadl E.M. Fadl Elmula has received speaker honorarium from Medtronic and Hemo Sapiens. Alexandre Persu, Yu Jin and Jan A. Staessen declare that they have no conflict of interest.
References
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373: 1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376: 1903–1909.
- Krum H, Schlaich MP, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629.
- Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al.; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401.
- Demaria AN. Reflections on renal denervation. J Am Coll Cardiol. 2014;63:1452–1453.
- Von Euler US, Hellner S, Purkhold A. Excretion of noradrenaline in urine in hypertension. Scand J Clin Lab Invest. 1954; 6:54–59.
- Julius S, Esler M. Autonomic nervous cardiovascular regulation in borderline hypertension. Am J Cardiol. 1975; 36:685–696.
- Esler M, Zweifler A, Randall O, Julius S, DeQuattro V. Agreement among three different indices of sympathetic nervous system activity in essential hypertension. Mayo Clin Proc. 1977;52:379–382.
- Eide I, Kolloch R, DeQuattro V, Miano L, Dugger R, Van der Meulen J. Raised cerebrospinal fluid norepinephrine in some patients with primary hypertension. Hypertension. 1979;1:255–260.
- Esler M, Jackman G, Leonard P, Bobik A, Skews H, Jennings G, et al. Determination of noradrenaline uptake, spillover to plasma and plasma concentration in patients with essential hypertension. Clin Sci (Lond). 1980;59 Suppl 6:311s–313s.
- Kjeldsen SE, Flaaten B, Eide I, Helgeland A, Leren P. Increased peripheral release of noradrenaline and uptake of adrenaline in essential hypertension? Clin Sci (Lond). 1981;61 Suppl 7:215s–217s.
- Kjeldsen SE, Zweifler AJ, Petrin J, Weder AB, Julius S. Sympathetic nervous system involvement in essential hypertension: Increased platelet noradrenaline coincides with decreased beta-adrenoreceptor responsiveness. Blood Press. 1994;3:164–171.
- Gifford RW. An algorithm for the management of resistant hypertension. Hypertension. 1988;11:I-171–I-175.
- Klein LE. Compliance and blood pressure control. Hypertension. 1988;11:I-161–I-164.
- Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34:87–90.
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al., for the LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359: 995–1003.
- Julius S, Kjeldsen SE, Weber M, Brunner H, Ekman S, Hansson L, et al. Cardiac events, stroke and mortality in high-risk hypertensives treated with valsartan or amlodipine: Main outcomes of The VALUE Trial. Lancet. 2004;363: 2022–2031.
- Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: Insights from meta-analysis of antihypertensive drug trials of 4,121 patients with focus on trial design: The CONVERGE report. Heart. 2013; 99:1579–1587.
- Shun-Shin MJ, Howard JP, Francis DP. Removing the hype from hypertension. Symplicity HTN-3 illustrates the importance of randomisation and blinding for exciting new treatments. BMJ. 2014;348:g1937.
- O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013; 31:1731–1768.
- Persu A, O’Brien E, Verdecchia P. Use of ambulatory blood pressure measurement in the definition of resistant hypertension: A review of the evidence. Hypertens Res. 2014 Apr 17. doi: 10.1038/hr.2014.83. [Epub ahead of print]
- Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, et al.; IDACO investigators. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Blood Press Monit. 2007;12:393–395.
- Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346.
- Azizi M, Steichen O, Frank M, Bobrie G, Plouin PF, Sapoval M. Catheter-based radiofrequency renal-nerve ablation in patients with resistant hypertension. Eur J Vasc Endovasc Surg. 2012;43:293–299.
- Persu A, Renkin J, Thijs L, Staessen JA. Renal denervation: Ultima ratio or standard in treatment-resistant hypertension. Hypertension. 2012;60:596–606.
- Staessen JA, Thijs L, Bieniaszewski L, O’Brien ET, Palatini P, Davidson C, et al. Ambulatory monitoring uncorrected for placebo overestimates long-term antihypertensive action. Systolic Hypertension in Europe (SYST-EUR) Trial Investigators. Hypertension. 1996;27:414–420.
- Lüscher TF, Mahfoud F. Renal nerve ablation after Symplicity HTN-3: Confused at the higher level? Eur Heart J. 2014; 35:1706–1711.
- Persu A, Azizi M, Burnier M, Staessen JA. Residual effect of renal denervation in patients with truly resistant hypertension. Hypertension. 2013;62:450–452.
- Fadl Elmula FEM, Hoffmann P, Fossum E, Brekke M, Gjønnæss E, Hjørnholm U, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532.
- Persu A, Renkin J, Asayama K, O’Brien E, Staessen JA. Renal denervation in treatment-resistant hypertension: The need for restraint and more and better evidence. Expert Rev Cardiovasc Ther. 2013;11:739–749.
- Persu A, Jin Y, Azizi M, Baelen M, Völz S, Elvan A, et al., on behalf of the European Network Coordinating research on REnal denervation (ENCOReD). Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2014;28:150–156.
- Helgeland A. Treatment of mild hypertension: A five year controlled drug trial. The Oslo study. Am J Medicine. 1980: 69:725–732.
- Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: Prospective case series. Hypertension. 2012;60:1485–1490.
- Fadl Elmula FEM, Hoffman P, Larstorp AC, Fossum E, Brekke M, Kjeldsen SE, et al. Adjusted drug treatment is superior to sympathetic renal denervation in patients with true treatment resistant hypertension. Hypertension. 2014;63:691–699.
- Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140.
- Ott C, Mahfoud F, Schmid A, Ditting T, Sobotka PA, Veelken R, et al. Renal denervation in moderate treatment-resistant hypertension. J Am Coll Cardiol. 2013;62:1880–1886.
- Jin Y, Persu A,Staessen JA. Letter by Jin et al. regarding article, “Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension”. Circulation. 2014;129:e499.
- Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, et al. ESH position paper: Renal denervation – An interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–841.
- Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, et al. Updated ESH position paper on interventional therapy of resistant hypertension. Euro Intervention. 2013;9:R58–R66.
- Schmieder RE. How should data from Symplicity HTN3 be interpreted? Nat Rev Cardiol. Advance online publication 20 May 2014; doi:10.1038/nrcardio.2014.70.
- Pathak A, Ewen S, Fajadet J, Honton B, Mahfoud F, Marco J, et al. From Symplicity HTN-3 to the Renal Denervation Global Registry: Where do we stand and where should we go? Euro Intervention. 2014;10:21–23.
- Longland CJ, Gibb WE. Sympathectomy in the treatment of benign and malignant hypertension; A review of 76 patients. Br J Surg. 1954;41:382–392.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension, results in 1,266 cases. JAMA. 1953; 152:1501–1504.
- Persu A, Jin Y, Baelen M, Vink E, Verloop WL, Schmidt B, et al.;European Network Coordinating research on REnal Denervation (ENCOReD) Consortium. Eligibility for renal denervation: Experience at 11 European expert centers. Hypertension. 2014;63:1319–1325.
- Persu A Jin Y, Fadl Elmula FEM, Jacobs L, Renkin J, Kjeldsen SE. Renal denervation after Symplicity HTN-3: An update. Curr Hypertens Rep. 2014;16:460. DOI 10.1007/s11906-014-0460-x.
- Oz M. Pressure relief. This year brings a breakthrough procedure to fight hypertension. Time Magazine. 2012, January 9, page 28.
- Blech J. Vergebens verbrutzelt. Ein Eingriff an den Nierennerven gilt als neues Wundermittel gegen Bluthochdruck. Dabei ist nicht bewiesen, dass die Methode den Patienten wirklich nutzt. Der Spiegel. 2013, No. 28:102–103.
- Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245.
