Abstract
Background. Migraine is a common type of primary headache predominantly seen in women. This study aimed to evaluate endothelial function in patients with migraine using pulse wave velocity (PWV). Methods. The study included 73 patients with newly diagnosed migraine and 80 healthy subjects. All patients and controls underwent baseline transthoracic echocardiography and PWV measurements. Patients were randomized to three groups to receive propranolol, flunarizine or topiramate, and the measurements were repeated at the end of 1 month. Results. The newly diagnosed migraine patients and the control group exhibited no differences in baseline clinical characteristics, and the measurements showed that PWV was 7.4 ± 1.0 m/s in the patient group and 6.0 ± 1.0 m/s in the control group (p < 0.001). The same measurements were repeated during a control visit at the end of 1 month. Following treatment, a significant decrease was observed in PWV in all patient groups compared to baseline (p < 0.001). Subgroup analysis showed significantly decreased PWV in all drug groups, with the most prominent decrease in the topiramate group. Conclusions. The increased PWV demonstrated in migraine patients in this study stands out as an additional parameter elucidating endothelial dysfunction in these patients. Decreasing the number of migraine attacks with prophylactic treatment may reduce PWV and decrease cardiovascular risk in long-term follow-up.
Introduction
Migraine is a risk factor for ischemic stroke, angina pectoris and myocardial infarction, and affects more than 14% of the general population, occurring predominantly among women (Citation1,Citation2). Migraine is characterized by recurrent episodes of one-sided, pulsatile, moderate to severe headache and it is usually accompanied by symptoms such as photophobia/phonophobia and nausea/vomiting with a duration of 4–72 h. According to the International Headache Society (IHS) classification, migraine is one of the primary headaches, and is generally defined as a neurovascular disorder associated with contractile dysfunction of cranial blood vessels, neurogenic inflammation and depression mechanisms spreading from the cerebral cortex. Various studies have shown that migraine is associated with neuroinflammatory conditions, cytokines, some neuropeptides and vasomotor changes; however, the pathogenesis of migraine has not been fully clarified (Citation3,Citation4).
Historically, the vascular changes in migraine have been considered to be limited to cranial vessels (Citation5). However, generalized peripheral vasoconstriction has been observed during migraine attacks (Citation6). Several studies have demonstrated coronary vasospasm, reduced endothelial function, decreased brachial artery compliance and increased peripheral arterial stiffness during the attacks in patients with migraine (Citation7–11).
Although the underlying mechanism of the relationship between migraine and cardiovascular diseases (CVDs) remains uncertain, studies have shown that the impaired functional properties of large arteries may be responsible for this relationship. Decreased arterial elasticity, i.e. increased arterial stiffness, has been shown to be a direct risk factor for CVDs (Citation12–15). Functional/structural stiffening of the arteries occurs via several mechanisms (Citation16,Citation17). Increased arterial wall tension causes functional arterial stiffening. In addition to endothelial dysfunction, elevated blood pressure, increased heart rate and/or sympathetic activation may increase functional arterial stiffness (Citation16,Citation18). Endothelial dysfunction occurs in the early stage of atherosclerotic vascular damage, and nitric oxide bioavailability in the endothelium is impaired (Citation19). Carotid-femoral pulse wave velocity (PWV) is the gold standard for assessing central arterial stiffness (Citation20). For this reason, PWV is also used as a marker of endothelial dysfunction (Citation19,Citation21). Endothelial dysfunction represents the highest risk among all risk factors for CVDs (Citation22).
Several methods are used to assess endothelial dysfunction, of which PWV is the most inexpensive, simple and reproducible method, and studies have found increased PWV in patients with migraine (Citation23,Citation24). However, no study has investigated the change in PWV following prophylactic treatment.
In this prospective study, we aimed to determine the difference in arterial stiffness values in prophylaxis-naive patients with migraine compared to the control group using carotid-femoral PWV. We investigated whether there was any change in arterial stiffness values in these patients with migraine following prophylaxis with propranolol, flunarizine or topiramate. Additionally, we determined which group had the most prominent change and whether this change was associated with the migraine attacks during prophylaxis, and we also investigated which drug group was most effective in preventing the attacks.
Subjects and methods
Study design and population
The study was conducted in the Department of Cardiology, Erciyes University, from November 2010 to September 2011. The study protocol was assessed and approved by the ethics committee of Erciyes University School of Medicine. Ninety patients in the age group 18–45 years, newly diagnosed with migraine according to IHS diagnostic criteria (Citation25) at the Neurology and Headache polyclinic between the aforementioned dates, who were eligible as per the inclusion and exclusion criteria mentioned below were included in this study. Five patients refused to participate in the study when the study protocol was explained. Six patients preferred to be excluded from the study, reasoning that they would be unable to participate owing to transportation and referral problems. Two patients were excluded from the study due to detection of serious valvular pathology during the cardiological tests performed as part of the study protocol. Four patients were excluded as they missed the control visits without giving a reason and could not be reached via the telephone numbers they provided. Finally, 73 patients who were diagnosed with migraine and met the eligibility criteria specified below were included in the study (). All of the patients were informed about the aim of the study and written informed consent was obtained from each patient.
Twenty-three of the study participants were male (31.5%) and 50 were female (68.5%). Inclusion criteria were as follows: patients who did not receive any drugs used jointly in migraine prophylaxis and for other conditions due to any other disease history within the past 3 months; patients with a history of at least two attacks per month who were indicated for prophylaxis at the discretion of a neurology specialist; for female patients, those between days 5 and 15 of the menstrual cycle; and patients who had no contraindications for the prophylaxis drugs.
Exclusion criteria were as follows: a history of CVD including hypertension, atrial fibrillation and valvular heart disease; patients with a high risk of coronary artery disease; patients with diabetes, impaired glucose tolerance, dyslipidemia, metabolic syndrome or thyroid disease; obese patients (body mass index ≥ 30 kg/m2); alcohol or heavy caffeine consumption; pregnancy or oral contraceptive use; and smoking.
Patients who were diagnosed with migraine at the Neurology and Headache polyclinic and included in the study completed the Henry Ford Hospital Headache Disability Inventory (HDI) and pre-treatment migraine-type headache survey, and detailed medical history was obtained from each patient. Neurological and systemic examinations were performed. Blood pressure and pulse rate were measured. Blood count, serum electrolyte levels, thyroid, liver and kidney function tests were performed. Cardiovascular examination and electrocardiography (ECG) were performed for each patient. The necessary evaluations were carried out to exclude the presence of any condition contraindicating the use of propranolol (a β-blocker), flunarizine (a calcium channel blocker) or topiramate (an antiepileptic drug) regarding cardiological, neurological or other aspects. As a result of these evaluations, patients with any inconvenient medical condition in terms of drug use were excluded from the study. Baseline transthoracic echocardiography (TTE) and PWV recordings were obtained for each patient.
A control group of 80 patients (25 males, 55 females), matched with the patient group in terms of gender, age, TTE parameters, blood pressure, heart rate and other baseline clinical characteristics, was included in the study. Accordingly, TTE and PWV were measured in this group as well.
The 73 patients who were indicated for migraine prophylaxis and included in the study were randomized to three groups. For this purpose, the first group received propranolol 40 mg 2 × 1, the second group received flunarizine 5 mg 2 × 1 and the third group received topiramate 50 mg 1 × 1 ().
Daily headache follow-up forms were given to the patients in each group. Following 1 month of medical treatment, all patients receiving prophylaxis were invited to a control visit, and the frequency, duration and severity of headaches were recorded, as well as drug use, side-effects of the drug and use of analgesics. Neurological and systemic examinations were performed. Blood pressure, heart rate, liver and kidney function tests were evaluated. TTE and PWV values were also measured and recorded during the same visit for the patients who received no treatment for acute attacks within the previous week. For the patients who had received analgesics during the past week, a control visit was recommended 1 week later. Similarly, for female patients who were not between days 5 and 15 of their menstrual cycle, control PWV and TTE values were measured and recorded on days corresponding to this specific period. The patients were interrogated about whether they had taken their medications regularly during the 1 month period, whether they had experienced any side-effects, the efficacy of the drugs and the number of migraine attacks.
Pulse wave velocity
Vascular studies were performed in the supine position at rest in a silent room with stable temperature (27°C). PWV was measured in all participants. Before carotid-femoral PWV measurement, the ECG leads of the device were connected to the patients. For device measurement calibration, the distance between the carotid and femoral arteries was measured with an elastic measure in addition to the patient chart information. The value was recorded on the device in millimeters.
For carotid-femoral PWV measurement, subsequent pulse wave recordings accompanied by ECG were obtained from the carotid artery, immediately followed by femoral regions. Measurements were performed with a PWV assessment device (Micro medical Pulse Trace, Rochester, UK) using carotid and femoral arteries. Transit time was calculated by measuring the duration between the R-wave in ECG and the pulse base in both regions, and the PWV value was obtained by dividing it by the total distance (m/s). Right femoral and right carotid arteries were used for all measurements. Signal uptake was recorded by a single blinded experienced operator, preferably after establishing 15 pulse wave throbs. The standard deviation value recommended by the manufacturer was used (SD < 5.1). PWV can be measured non-invasively and the technique has been found to be extremely reproducible, with replicate testing yielding a correlation > 0.80 (Citation26).
Echocardiography
All participants were evaluated with a GE-Vingmed Vivid 7 system echocardiography device using a 2.5 MHz transducer. According to the American Society of Echocardiography guidelines (Citation27), patients were assessed in the left lateral decubitus position and measurements were performed using two-dimensional, M-mode color Doppler accompanied by parasternal long axis view, short axis view, apical four chamber and five chamber views. At least three subsequent pulses in sinus rhythm were recorded and averaged by a single blinded experienced operator.
Positioning the M-mode cursor right below the mitral leaflets in the parasternal long axis view, left ventricular diameters [left ventricular systolic and diastolic diameters (LVSD and LVDD)], interventricular septum (IVS) and posterior wall (PW) thickness, and left ventricular ejection fraction (EF) were measured according to the American Society of Echocardiography guidelines (Citation27).
Measurement of carotid intima-media thickness
The method was based on the technique developed by Pignoli et al. (Citation28), using a linear B-mode 7.5 MHz ultrasound transducer. The carotid intima-media thickness (CIMT) was measured by two different radiologists together, who were unaware of the clinical data of the patient, and a consensus was achieved for the measurements. On a longitudinal, two- dimensional ultrasound image of the carotid artery, the posterior wall of the carotid artery was displayed as two bright white lines separated by a hypoechogenic space. The distance between the leading edge of the first bright line of the far wall (lumen interface) and the leading edge of the second bright line (media adventitia interface) indicated the CIMT. The CIMT was measured in the far (deeper) wall of left distal common carotid artery. The carotid artery segment 1 cm proximal to the bifurcation was regarded as the reference for measurements. When an optimal image was obtained, it was frozen on the R-wave of the ECG and at least five measurements were taken. After excluding the maximum and minimum values, the arithmetical average of the remaining three measurements was calculated automatically and used for statistical analysis.
Blood pressure measurement
Blood pressure was measured in the sitting position after resting for at least 10 min, twice from the right arm with 2 min intervals using the MEC 1000 patient monitoring device and the oscillometric method. The average value of the two measurements was calculated and recorded. According to the 7th Report of the Joint National Committee, hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and diastolic blood pressure (DBP) ≥ 90 mmHg (Citation29).
Statistical analysis
Parametric variables were expressed as mean ± SD, and non-parametric variables as median, minimum and maximum values. For the continuous variables in intergroup comparisons, parametric tests including independent-samples t test, paired-samples t test, one-way analysis of variance and Tukey's HSD method, and non-parametric tests including the Mann-Whitney U test and Wilcoxon test were used. The chi-squared test was used for the comparison of categorical variables. Correlation analysis was performed using Spearman's correlation method. The results of the analyses were assessed within the 95% confidence interval, and p < 0.05 was considered a statistically significant difference. SPSS 15.0 software (Version 15; SPSS, Chicago, IL, USA) was used for the main statistical analysis.
Results
The present study included 73 patients (50 females, 23 males; mean age 32.8 years) newly diagnosed with migraine. In addition, 80 healthy participants (55 females, 25 males; mean age 33.8 years) were included as the control group. The two groups were similar in terms of clinical and demographic characteristics (age, gender, LVSD, LVDD, IVS, PW, EF, SBP, DBP and heart rate) (p > 0.05) (). As hypertension, diabetes, impaired glucose tolerance, dyslipidemia, metabolic syndrome, obesity, oral contraceptive use and smoking were exclusion criteria, there were no significant differences in cardiovascular risk factors between the patient and control groups (p > 0.05).
Table I. Demographic, echocardiographic and clinical features of migraine patients and healthy subjects.
PWV was 7.4 ± 1.0 m/s in the group of patients with migraine, while it was 6.0 ± 1.0 m/s in the control group. There was a significant difference between the two groups in terms of PWV (p < 0.001) (). Comparison of the CIMT of the two groups revealed greater CIMT in patients with migraine; however, this difference was not statistically significant (p = 0.073) ().
The patients who were indicated for migraine prophylaxis and included in the study were randomized to three groups. The first of these groups received propranolol 40 mg 2 × 1, the second group received flunarizine 5 mg 2 × 1 and the third group received topiramate 50 mg 1 × 1. There was no significant difference among the three groups in terms of age, gender, LVSD, LVDD, IVS, PW, EF, SBP, DBP, heart rate and CIMT (0.6, 0.9, 0.1, 0.4, 0.3, 0.5, 0.1, 0.1, 0.7, 0.09 and 0.8, respectively) (). TTE, CIMT, PWV and SBP/DBP measurements were repeated during the control visit at the end of 1 month.
Table II. Comparison of baseline characteristics in the three prophylactic drug groups.
Compared to baseline values, no significant change was observed in the LVSD, LVDD, IVS, PW, EF, DBP and CIMT of patients during the control measurements (p > 0.05).
When pre-prophylaxis and post-prophylaxis values of PWV were compared, a significant decrease was found in post-treatment PWV values (7.4 ± 1.0 vs 6.7 ± 1.0 m/s, p < 0.001) (, ). Comparison of pre- and post-prophylaxis SBP values showed a similar decrease after treatment (118.6 ± 10.5 vs 115.3 ± 10.8 m/s, p = 0.005) (). When male and female patients were compared in terms of PWV improvement, no significant difference was found between the two groups (p = 0.32).
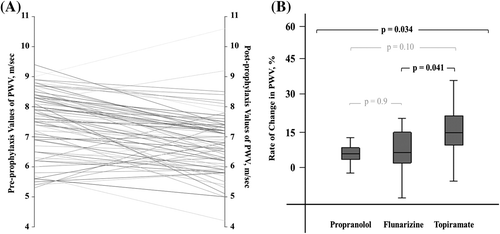
Table III. Comparison of pre- and post-prophylaxis in terms of systolic blood pressure (SBP) and pulse wave velocity (PWV) values in patients with migraine.
When the differences in PWV measurements were evaluated by drug group, all three drugs were found to provide significant improvement in PWV values (). Comparison of the PWV-lowering effects of these drugs revealed a significant difference between the three groups (p = 0.034) (). When the drugs were compared against one another, a significant difference was found between the PWV-lowering effects of flunarizine and topiramate (p = 0.041) (). The β-blocker used in this study, propranolol, significantly decreased SBP values (). Flunarizine and topiramate had no significant effects on SBP ().
Analyses showed that the prophylaxis administered to patients with migraine did not reduce the PWV values to the levels seen in normal healthy subjects. When drug subgroups were evaluated, levels in those receiving topiramate prophylaxis were found to be decreased to the levels seen in normal healthy subjects.
In patients with migraine, the number of migraine attacks that developed during the 1 month prophylaxis was recorded at the control visit. Following the interrogation about migraine attacks, the success of prophylaxis was assessed based on the threshold of one or no attack by dividing the patients into “one or no attack” and “more than one attack” groups. During the 1 month period, 75% of patients receiving propranolol (18/24 patients), 71% of patients receiving flunarizine (17/24) and 96% of patients receiving topiramate (24/25) reported one or no attack (). Comparison of the three drug groups in terms of prophylaxis efficacy revealed no significant difference (p = 0.052), and paired comparisons demonstrated no significant difference between propranolol and flunarizine (p = 0.74). However, topiramate was seen to be significantly superior compared to flunarizine and propranolol in terms of prophylaxis efficacy, although the difference was borderline for the latter (p = 0.023 and 0.049, respectively).
Table IV. Development of migraine attacks during the prophylaxis treatment.
A negative and significant correlation was found between the change in PWV and the number of migraine attacks during 1 month (r = −0.42, p < 0.001).
Discussion
In the present study we have measured PWV, which is used to assess arterial stiffness, and demonstrated significantly increased arterial stiffness in patients with migraine compared to the control group. The increased PWV values in patients with migraine indicated endothelial dysfunction, and may explain the increased risk of CV events, which has not been clarified in previous studies (Citation30–33).
Arterial stiffness is a valuable aspect in predicting CVD and mortality (Citation15,Citation34). The gold standard in determining arterial stiffness is measuring carotid-femoral PWV (Citation35). Advantages of this method include the non-invasive nature, widespread use, highly accurate results and reproducibility of the test.
In the first study using PWV measurement in young patients with migraine, Schillaci et al. showed significantly increased augmentation index and PWV parameters compared to matched controls (Citation23). Some studies have suggested that patients with migraine have increased CVD risk factors compared to normal healthy subjects, and these increased CVD risk factors have been considered responsible for the increased arterial stiffness and ischemic vascular events (Citation31,Citation33). Conversely, Schillaci et al. showed that increased PWV could not be the only reason underlying the CVD risk factors in patients with migraine (Citation23).
Ikeda et al. also designed a study in migraine patients without risk factors for CVD compared to healthy subjects, and found significantly higher PWV levels in patients with migraine (Citation24).
Yetkin et al. demonstrated endothelial and vascular dysfunction in patients with migraine in two different studies (Citation8,Citation9). In addition to its association with migraine, endothelial dysfunction has been associated with obesity, increased insulin resistance, hypertension, dyslipidemia, ischemic stroke in young adults and coronary artery disease (Citation36–41). The presence of significant associations between these disorders and their relationship with migraine support the hypothesis that endothelial and vascular dysfunction may underlie the pathophysiology of all of these disorders (Citation30–33,Citation36,Citation42). The increased rate of ischemic stroke and acute coronary syndromes among patients with migraine, and the fact that PWV is increased in both the aforementioned disorders and metabolic conditions as well as migraine, also support this possibility.
Substances synthesized by the endothelium are involved in essential activities determining vascular tonus and permeability such as hemostasis, inflammatory response and angiogenesis. Disruption of this balance results in endothelial dysfunction (Citation43). The thrombogenic and inflammatory environment resulting from endothelial dysfunction plays an important role in the pathophysiology of atherosclerosis and hypertension, and this has been demonstrated in previous studies (Citation43,Citation44). Similarly, inflammation, oxidative stress, disrupted aggregation and thrombosis tendency have also been demonstrated in patients with migraine (Citation45–47).
Tietjen et al. conducted a study in young female patients with migraine and obtained results consistent with endothelial activation, which is part of the endothelial dysfunction seen in patients with migraine compared to the control group. They suggested that endothelial dysfunction may be “the highest risk among cardiovascular risk factors” and that the statins, angiotensin-converting enzyme inhibitors, calcium channel blockers and β-blockers used in the treatment of migraine may improve inflammation, thrombocyte function, vascular endothelium and cardiovascular risk through their endothelial function correcting effects (Citation48). However, there are no comprehensive studies demonstrating this hypothesis. Because prophylactic migraine treatment aims to reduce migraine attacks rather than to improve endothelial function, and because the treatment is frequently interrupted by the physician or the patient, the favorable effects of these drugs on endothelial function have been overlooked and not clearly evaluated in patients with migraine.
In the present study, we found reduced headache and a more profound improvement in PWV with topiramate compared to the other drugs, and we also observed that the decreased number of migraine attacks provided more improvement in PWV in all migraine patients. There was a profound increase in arterial stiffness values among the patients in the topiramate group who experienced less headache as well as those with reduced attack recurrence across the entire patient group during the prophylaxis. This study also shows that endothelial function is unfavorably affected by each attack, and inhibition of the attacks improves vascular function. These results support the hypothesis that migraine is an inflammatory disorder, with each migraine attack triggering systemic inflammation as well as subsequent endothelial dysfunction, and the systemic inflammation and endothelial dysfunction may be causing the increased coronary artery disease and stroke rates among patients with migraine.
The most important limitation of the present study is that no distinction was made between migraine patients with and without aura. Some studies have shown a significant difference between migraine patients with and without aura regarding ischemic stroke and ischemic vascular events (Citation23,Citation49). However, the study that Kurth et al. conducted in female patients with migraine did not demonstrate an increased risk of ischemic vascular events in migraine patients without aura (Citation2). The second limitation is that the duration of migraine was not recorded in the present study. Recurrent migraine attacks lead to further inflammation and endothelial dysfunction, and the degree of dysfunction cannot be determined without knowing the duration of the disease. The fact that there was no improvement in PWV values in some patients despite prophylaxis in the present study may be associated with the duration of migraine and the number of migraine attacks. The control measurements obtained after 1 month of prophylaxis given to the patients appear to be another limitation. The final limitation in our study is that we did not use other markers indicating arterial stiffness, such as flow-mediated dilatation (FMD), salbutamol plus central pressure or biochemical markers, because of economic problems.
In conclusion, the degree of arterial stiffness in patients with migraine may be used as an effective parameter providing information about the severity of the disease and guidance for treatment. Effective prophylaxis of migraine may improve arterial stiffness and the related endothelial functions, and provide favorable contributions regarding improved morbidity and mortality among these patients in the long term. Comprehensive studies with long-term follow-up are required on this subject.
Declaration of interest: The authors have no conflicts of interest.
References
- Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–91.
- Hamed SA. The vascular risk associations with migraine: relation to migraine susceptibility and progression. Atherosclerosis. 2009;205:15–22.
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine - current understanding and treatment. N Engl J Med. 2002;346: 257–70.
- Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24 Suppl 2:2–7.
- Iversen HK, Nielsen TH, Olesen J, Tfelt-Hansen P. Arterial responses during migraine headache. Lancet. 1990;336:837–9.
- Lafitte C, Even C, Henry-Lebras F, de Toffol B, Autret A. Migraine and angina pectoris by coronary artery spasm. Headache. 1996;36:332–4.
- Yetkin E, Ozisik H, Ozcan C, Aksoy Y, Turhan H. Decreased endothelium-dependent vasodilatation in patients with migraine: a new aspect to vascular pathophysiology of migraine. Coron Artery Dis. 2006;17:29–33.
- Yetkin E, Ozisik H, Ozcan C, Aksoy Y, Turhan H. Increased dilator response to nitrate and decreased flow-mediated dilatation in migraineurs. Headache. 2007;47:104–10.
- de Hoon JN, Willigers JM, Troost J, Struijker-Boudier HA, van Bortel LM. Cranial and peripheral interictal vascular changes in migraine patients. Cephalalgia. 2003;23:96–104.
- Nagai T, Tabara Y, Igase M, Nakura J, Miki T, Kohara K. Migraine is associated with enhanced arterial stiffness. Hypertens Res. 2007;30:577–83.
- Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140: 669–82.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43.
- Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–6.
- Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–9.
- Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010;74:24–33.
- Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, et al. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol. 1998; 32:123–7.
- Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro Moraes C, et al. Vascular stiffness and endothelial dysfunction: correlations at different levels of blood pressure. Blood Press. 2012;21:31–8.
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75.
- Schillaci G, Sarchielli P, Corbelli I, Pucci G, Settimi L, Mannarino MR, et al. Aortic stiffness and pulse wave reflection in young subjects with migraine: a case-control study. Neurology. 2010;75:960–6.
- Ikeda K, Hirayama T, Iwamoto K, Takazawa T, Kawase Y, Yoshii Y, et al. Pulse wave velocity study in middle-aged migraineurs at low cardiovascular disease risk. Headache. 2011;51:1239–44.
- Olesen J, Steiner TJ. The international classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry. 2004;75:808–11.
- Sutton-Tyrrell K, Mackey RH, Holubkov R, Vaitkevicius PV, Spurgeon HA, Lakatta EG. Measurement variation of aortic pulse wave velocity in the elderly. Am J Hypertens. 2001; 14:463–8.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–406.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72.
- Bigal ME, Gironda M, Tepper SJ, Feleppa M, Rapoport AM, Sheftell FD, et al. Headache prevention outcome and body mass index. Cephalalgia. 2006;26:445–50.
- Cirillo M, Stellato D, Lombardi C, De Santo NG, Covelli V. Headache and cardiovascular risk factors: positive association with hypertension. Headache. 1999;39:409–16.
- Schwaag S, Nabavi DG, Frese A, Husstedt IW, Evers S. The association between migraine and juvenile stroke: a case-control study. Headache. 2003;43:90–5.
- Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005; 64:614–20.
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111: 3384–90.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
- Galvao R, Plavnik FL, Ribeiro FF, Ajzen SA, Christofalo DM, Kohlmann O Jr. Effects of different degrees of insulin sensitivity on endothelial function in obese patients. Arq Bras Cardiol. 2012;98:45–51.
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–7.
- Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87:1051–7.
- Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–6.
- Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109: 184–9.
- Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–82.
- Scher AI, Lipton RB, Stewart W. Risk factors for chronic daily headache. Curr Pain Headache Rep. 2002;6:486–91.
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32.
- Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–58.
- Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–7.
- Tietjen EG. Migraine and ischaemic heart disease and stroke: potential mechanisms and treatment implications. Cephalalgia. 2007;27:981–7.
- Hamed SA, Hamed EA, Ezz Eldin AM, Mahmoud NM. Vascular risk factors, endothelial function, and carotid thickness in patients with migraine: relationship to atherosclerosis. J Stroke Cerebrovasc Dis. 2010;19:92–103.
- Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA. Migraine and biomarkers of endothelial activation in young women. Stroke. 2009;40:2977–82.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, d’Anglejan-Chatillon J, et al. Case-control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310:830–3.