Abstract
Objective. To investigate the oxidative profile and thrombotic markers in obese and hypertensive mothers. Methods. Thirty obese, 28 hypertensive and 34 healthy control mothers were recruited from Tlemcen Hospital, Algeria. Plasma vitamin C, nitric oxide, superoxide anion, erythrocyte glutathione, malondialdehyde, carbonyl proteins and erythrocyte antioxidant enzyme activities and coagulation markers [protein C, protein S, fibrinogen, prothrombin, antithrombin, activated partial thromboplastin time (APTT), lupus anticoagulants (LACs)] were measured. Changes in plasma urea, creatinine, uric acid, glucose and lipid levels were also determined. Results. Plasma glucose concentrations were high in obese mothers, and plasma urea, uric acid and creatinine levels were increased in hypertensive compared with healthy mothers. Obese and hypertensive mothers had low vitamin C and glutathione values, catalase and superoxide dismutase activities, and high triglyceride, superoxide anion, malondialdehyde and carbonyl protein levels compared with control mothers. Plasma nitric oxide levels were enhanced in obese mothers but reduced in hypertensive mothers. Fibrinogen and prothrombin levels were significantly enhanced in obese and hypertensive mothers. Protein C, protein S, antithrombin and APTT values were significantly higher in hypertensive mothers. Only hypertensive mothers were positive for LACs. Conclusion. Obese and hypertensive mothers presented oxidative stress and a pro-thrombotic state. Their oxidative and hemostasis profile should be carefully considered and appropriate management organized.
Introduction
Endocrine, metabolic and vascular changes are observed during pregnancy to provide sufficient energy and nutrients to the fetus. The coagulation system is activated during pregnancy, leading to a state of hypercoagulability, which predisposes to the development of thrombosis (Citation1). This hypercoagulability state is a normal physiological mechanism to prevent post-partum hemorrhage. Concentrations of fibrinogen, prothrombin, thrombin and thrombin–antithrombin (TAT) complexes are increased, while anticoagulant levels such as protein S are decreased during normal pregnancy. However, the other major anticoagulants, protein C and antithrombin III, are relatively unchanged (Citation2). Fibrinolysis is impaired by an increase in plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2). Hypercoagulability during pregnancy could be related to acquired factors such as antiphospholipid antibodies or to congenital ones like prothrombin mutation, protein C, protein S and antithrombin deficiencies (Citation1). Also, pregnancy can cause hypercoagulability by other factors such as cesarean section, maternal age greater than 35 years, multigravidity and several pregnancy complications such as preeclampsia, hypertension and obesity (Citation3).
On the other hand, pregnancy is a condition exhibiting increased susceptibility to oxidative stress because of high oxygen requirements and the mitochondrion-rich placenta. Oxidative stress markers are increased in normal pregnancy (Citation4). Pregnancy is an oxidative state characterized by placental production of superoxide anion (O2–.) and hydrogen peroxide (H2O2). In normal pregnancy, the production of these reactive radicals is accompanied by their elimination by antioxidant defenses (Citation4). However, in complicated pregnancies such as pregnancy-induced hypertension and obesity, free radical production exceeds the antioxidant capacity, leading to an exaggerated oxidative stress (Citation5,Citation6).
Maternal obesity has emerged as an important risk factor associated with pregnancy complications and fetal growth abnormalities. Obese pregnant women have increased inflammation and oxidative stress, and low levels of antioxidant defenses, which contribute to adverse pregnancy outcomes (Citation5,Citation7). The incidence of venous thromboembolism during pregnancy is high in obese women because of pregnancy-related venous stasis, vascular damage and physiological hypercoagulability (Citation8).
Hypertension, especially when complicated by preeclampsia, is a major cause of maternal and fetal morbidity and mortality due to endothelial dysfunction, reduced maternal–fetal blood flow, inadequate supply of nutrients and intrauterine growth retardation (IUGR) (Citation9). Gestational hypertension is characterized by several lipid alterations which may contribute to endothelial dysfunction in preeclampsia (Citation10,Citation11). In gestational hypertension and preeclampsia, an imbalance of lipid peroxidation and antioxidant mechanisms, such as increased lipid peroxidation and diminished antioxidant capacity, could also impair endothelial function (Citation6,Citation12). Preeclampsia is by itself a highly thrombotic and procoagulant state, with platelet activation and increased concentration of total fibrinogen (Citation13,Citation14).
During the association of pregnancy and an additional hypercoagulable or oxidative state, the risk of thrombosis or oxidative stress may become substantial. Under pro-thrombotic conditions, the hemostatic response could lead to ischemic conditions, consequent generation of reactive oxygen species (ROS) and inflammation (Citation15). A series of events initiated by oxidative stress leads to several responses including vasoconstriction, membrane oxidation, further pro-coagulation and further generation of ROS (Citation16). All these reactions imply a potential relation between pro-thrombotic conditions in pregnancy and oxidative stress, which has not been previously clearly linked in pregnancies complicated by hypertension or by obesity. Nevertheless, the simultaneous existence of an oxidative stress and a pro-thrombotic profile during maternal obesity or hypertension is still not well understood. In addition, to our knowledge, there are no previous studies reporting thrombosis as well as oxidative markers in obese and hypertensive pregnant women.
The aim of this study was to identify thrombotic and oxidative stress markers in hypertensive and obese pregnant women. The purpose of this study is to improve the management of hypertensive and obese women during pregnancy.
Materials and methods
Patients
Pregnant women (n = 92) were selected and recruited at the Gynecology and Obstetrics Department of Tlemcen Hospital, Algeria. This case–control study was carried out with their written consent. The experimental protocol was approved by the Tlemcen Hospital Committee for Research on Human Subjects and was conducted in accordance with the Declaration of Helsinki.
Three groups were selected and studied: group 1 consisted of 34 non-obese and normotensive mothers (control group), group 2 consisted of 30 obese mothers [pregravidic body mass index (BMI) > 30 kg/m2] and group 3 consisted of 28 hypertensive mothers. Gestational hypertension was defined as an increase in systolic blood pressure of 30 mmHg or higher and/or an increase of diastolic blood pressure of 15 mmHg or higher from average values before 20 weeks’ gestation. In this study, all hypertensive women had proteinuria greater than 0.5 g/24 h, which was a characteristic of preeclampsia.
Exclusion criteria were chronic hypertension, diabetes or renal and cardiovascular diseases, previous thromboembolic complications, use of any kind of vitamin K antagonist or anticoagulant drugs and use of antioxidant supplements. Care was taken to match the age and week of pregnancy of the healthy pregnant group and the obese or hypertensive group. The rigorous selection, recruitment and monitoring of pregnant women was done by clinicians of the service.
Blood samples
Fasting maternal blood samples were obtained from the antecubital veins of the mothers, within 48 h of birth, and placed into test-tubes containing 1 ml of 3.8% sodium citrate. After centrifugation of these specimens for 10 min at room temperature and 2500 × g, the plasma specimens were placed into 1.5 ml tubes and kept at –80°C in the deep freezer until analysis.
Biochemical analysis
Plasma glucose, cholesterol, triglyceride, creatinine, uric acid and urea were measured using enzymatic colorimetric methods (kits from BioAssay Systems, CA, USA). For these enzymatic methods, the intra-assay coefficient of variation (CV) was < 2.5% and the inter-assay CV was in the range of 1.7–3%.
Oxidant/antioxidant markers
Plasma nitric oxide (NO) was determined by the colorimetric method of Griess (Sigma Aldrich Kit, St Louis, MO, USA), with an intra-assay CV of 2.5% and an inter-assay CV of 3.4%.
The determination of plasma O2–. was based on nitroblue–tetrazolium (NBT) reduction in monofarmazan by O2–. (Citation17). The assay mixture consisted of 50 mM Tris–HCl buffer (pH 8.6), 0.1 mM EDTA, 0.1 mg/ml gelatin and 0.1 mM NBT. The reaction was started by the addition of plasma, in 96-well flat-bottomed microtiter plates (Nunc, Paris, France). For the blank probe, an adequate volume of distilled water was used instead of plasma. The absorbance was recorded every 30 s during the first 2 min of the reaction at 560 nm. The blue formazan formed was dissolved using 2 M potassium hydroxide and dimethyl sulfoxide. The molar extinction coefficient (1.5 × 104 M–1 cm–1) was used to calculate the concentration of O2–.. The intra-assay CV was 2.5% and the inter-assay CV was 3.3%.
Erythrocyte malondialdehyde (MDA) levels, a marker of lipid peroxidation, were determined by the reaction of MDA with thiobarbituric acid (Sigma Aldrich, St Louis, MO, USA), based on the method of Draper and Hadley (Citation18), with an intra-assay CV of 2.5% and an inter-assay CV of 3%. MDA reacts with thiobarbituric acid to form a colorimetric product, proportional to the MDA present. Absorbance was measured at 532 nm.
Erythrocyte carbonyl proteins, a marker of protein oxidation, were assayed using 2,4-dinitrophenylhydrazine (DNPH) (Sigma Aldrich, St Louis, MO, USA), based on the method of Levine et al. (Citation19), with an intra-assay CV of 0.4% and an inter-assay CV of 0.6%. The derivatization of protein carbonyl groups with DNPH leads to the formation of stable dinitrophenyl (DNP) hydrazone adducts, which can be detected spectrophotometrically at 375 nm. Oxidized bovine serum albumin standard was used for the standard curve.
Erythrocyte reduced glutathione (GSH) levels were assayed by a colorimetric method based on the use of 5,5-dithiobis-(2-nitrobenzoic) acid (DTNB), according to a Sigma Aldrich kit (St Louis, MO, USA), with an intra-assay CV of 2.5% and an inter-assay CV of 3.6%. The lysates were first deproteinized with 5% 5-sulfosalicylic acid solution, centrifuged to remove the precipitated protein and then assayed for GSH. The measurement of GSH uses a kinetic assay in which catalytic amounts of GSH cause a continuous reduction of DTNB to TNB. The yellow product, 5-thio-2-nitrobenzoic acid (TNB), is measured spectrophotometrically at 412 nm.
Erythrocyte catalase (CAT, EC 1.11.1.6) activity was measured by the Cayman Chemical Catalase Assay Kit (Caymen Chemical Company, MI, USA), with an intra-assay CV of 2.4% and an inter-assay CV of 3%. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced is measured spectrophotometrically with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole as the chromogen.
Erythrocyte superoxide dismutase (SOD) activity was assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine, according to a Caymen kit (Caymen Chemical Company, MI, USA), with an intra-assay CV of 3% and an inter-assay CV of 3.8%.
Plasma vitamin C levels were estimated using Folin phenol reagent (Citation20), with an intra-assay CV of 2.6% and an inter-assay CV of 3.4%.
Thrombosis markers
Protein C (Chromogenix, Milan, Italy), protein S (Chromogenix, Milan, Italy), prothrombin (Chromogenix, Milan, Italy) and antithrombin (Chromogenix, Milan, Italy) activity was determined quantitatively by a coagulation analyzer, with an intra-assay CV of < 2.4% and an inter-assay CV in the range of 2–3.4%.
Fibrinogen concentrations were determined using the commercially Assay Max Human Fibrinogen enzyme-linked immunosorbent assay (ELISA) kit (BioCat, Heidelberg, Germany), as per the manufacturer's instructions. This assay employs a quantitative competitive enzyme immunoassay technique using specific polyclonal antibody, streptavidin–peroxidase conjugate and a peroxidase enzyme substrate. The intra-assay CV was 3.6% and the inter-assay CV was 4.8%.
Activated partial thromboplastin time (APTT) was measured using standard APTT kit reagents (No. 12203; Diagnostica Stago, Parsippany, NJ, USA) on the coagulation analyzer according to the manufacturer's instructions. The intra-assay CV was 2.2% and the inter-assay CV was 3.4%.
Lupus anticoagulants (LACs) were screened using a LAC-sensitive APTT reagent, PTT-LA (Diagnostica Stago, Asnieres, France). Tests were performed according to the manufacturer's recommendations. PTT-LA is a reagent containing cephalin prepared from rabbit cerebral tissue with a siliceous activator. Results were presented as the clotting times of the tested plasma samples. The intra-assay CV was 2.4% and the inter-assay CV was 3.1%.
LACs were also determined by a dilute Russell viper venom time (DRVVT) test, using a kit containing LA-1 (screening reagent) and LA-2 (confirmatory reagent) (Diagnostica Stago, Asnieres, France) according to the manufacturer. The LA-1 screening reagent contains Russell viper venom, phospholipids, antiheparin agents, calcium, buffers and stabilizers, to directly activate factor X. The LA-2 confirmation reagent is a phospholipid-rich reagent, to confirm the phospholipid-dependent nature of an inhibitor. After adding LA reagents to plasma, clot formation was automatically measured. With the LA-1 screening test, if the result of the DRVVT is within the normal range, the screening test is considered negative for LA. Samples showing a prolongation of the DRVVT with the LA-1 reagent were studied further by repeating the DRVVT using the LA-2 reagent. The intra-assay CV was 2.7% and the inter-assay CV was 3.2%.
All tests were performed on the BCS automated coagulation analyzer (Dade Behring, Marburg, Germany) at the Hemobiology Department of Tlemcen Hospital.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using STATISTICA (version 4.1; StatSoft, Paris, France). All variables were checked for normal distribution using the Shapiro–Wilk's test. Differences between groups were tested using unpaired Student's t tests for two groups and one-way analysis of variance (ANOVA) for more groups. The ANOVA test was followed by Tukey's post hoc test. Statistical significance was set as p values < 0.05.
Results
The clinical characteristics of the mothers are summarized in . All subjects were of similar age, gestational age and parity. We observed that the systolic and diastolic blood pressure were significantly higher in hypertensive mothers than in healthy subjects. The mean BMI values were significantly increased in obese mothers compared with controls. In addition, birth weight was significantly increased in the obese group and significantly reduced in the hypertensive group compared with the control group.
Table I. Maternal and neonatal characteristics.
Maternal biochemical parameters
Significant differences were found between obese and control subjects for plasma glucose levels, which were high in obese mothers (). Plasma urea, creatinine and uric acid concentrations were not significantly altered in obese mothers compared with controls. However, these parameters were significantly higher in hypertensive mothers compared with healthy mothers. Plasma triglyceride values were increased in obese and hypertensive mothers compared with control mothers. No significant difference in plasma cholesterol concentration was found between the obese, hypertensive and control mothers.
Table II. Maternal biochemical parameters.
Maternal oxidative stress biomarkers
Erythrocyte MDA and carbonyl protein levels and plasma O2–. concentrations were increased in obese and hypertensive mothers compared with control values; the highest values were seen in hypertensive mothers (). Plasma NO levels were enhanced in obese mothers but reduced in hypertensive mothers compared with controls.
Table III. Maternal oxidative stress parameters.
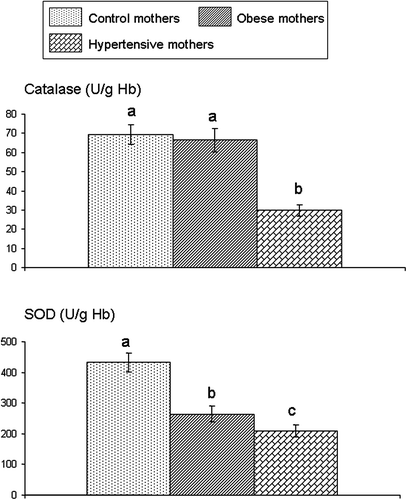
Plasma vitamin C levels and erythrocyte GSH concentrations were lower in obese and hypertensive mothers than in control mothers; the lowest values were seen in hypertensive mothers (). While erythrocyte catalase activity did not differ significantly between obese and control mothers, this activity was significantly lower in hypertensive mothers than in controls (). Erythrocyte activity of SOD was decreased in obese and hypertensive mothers.
Maternal thrombosis markers
Fibrinogen and prothrombin levels were significantly enhanced in obese and hypertensive mothers compared with control values; the highest values were seen in hypertensive mothers ().
Table IV. Maternal thrombosis parameters.
While no significant difference was found between obese and control mothers, the protein C and protein S values were significantly lower and antithrombin values were higher in hypertensive mothers compared with controls.
The APTT was found to be significantly prolonged in hypertensive patients than in controls. Nevertheless, there was no significant difference between the healthy and the obese mothers.
Using the PTT-LA and DRVVT as screening tests for LACs, we observed that only hypertensive mothers were positive for LACs. The PTT-LA and the DRVVT were prolonged in hypertensive mothers compared with controls. In contrast, in obese mothers, these values were similar to those of control mothers.
Discussion
This study provides evidence for a significant oxidative perturbation in both obese and hypertensive mothers, directly related to a pro-thrombotic status. These findings support a relationship between oxidant stress and thrombosis in complicated pregnancies.
In our study, the birth weight of newborns of obese mothers was significantly increased compared with control newborns. Clinical studies indicate that maternal obesity mainly results in fetal macrosomia (Citation5,Citation21). In contrast, the birth weight of newborns of hypertensive mothers was significantly decreased compared with control newborns. The association between maternal hypertension and IUGR is well documented (Citation22).
In our study, all obese mothers had normal plasma urea, creatinine and uric acid levels compared with control values, since all were normotensives and had normal renal function. Obese mothers had high plasma triglyceride concentrations, while total cholesterol values were unchanged compared with control values, in agreement with previous studies (Citation5,Citation23). Hypertriglyceridaemia is well known in obese subjects and can be attributed to enhanced hepatic very low-density lipoprotein (VLDL) and triglyceride production; reduced adipose tissue lipoprotein lipase activity, which restrains VLDL removal from the circulation; and excess adipose tissue, which exposes the liver to high concentrations of free fatty acids, leading to increased hepatic triglyceride production and secretion.
Hypertensive mothers had increased plasma urea, creatinine and uric acid levels compared with control values. Plasma creatinine and urea are commonly elevated in hypertension. Uric acid levels were found to be high in preeclampsia (Citation24).
Hypertensive mothers also showed high triglyceride concentrations and normal cholesterol levels compared with control mothers, in agreement with previous studies (Citation10,Citation11).
Our data revealed that the oxidant/antioxidant balance was altered in both obese and hypertensive mothers. We noted increased oxidative stress markers such as erythrocyte MDA and protein carbonyl levels in obese mothers. Elevated levels of oxidant markers in obese patients could result from their hyperglycemia, abnormal metabolism and metabolites in adipose tissue and/or excessive proinflammatory and inflammatory cytokine release and decreased antioxidant status (Citation25). MDA and carbonyl proteins are commonly used as indicators of lipid peroxidation and protein oxidation. MDA and carbonyl proteins were found to be high in obesity (Citation5). In addition, superoxide anion levels were higher in obese mothers than in control mothers. Superoxide anion concentrations have been reported to be increased in obese pregnancies (Citation23).
Oxidative stress contributes significantly to endothelial dysfunction in cardiovascular disease, as superoxide radicals readily inactivate NO, thereby impairing vasorelaxation. In our study, NO levels were increased in obese mothers, in agreement with previous findings (Citation23). NO is generated from the oxidation of arginine by nitric oxide synthase and acts as a vasodilator. During pregnancy, NO is one of the most important relaxing factors for the myometrium and in the control of blood flow in the uterus and placenta. Although beneficial effects of physiological concentrations of NO are established, high NO levels are reported in several diseases, including metabolic syndrome and obesity (Citation26).
In our study, in obese mothers, high pro-oxidant markers were associated with low antioxidant markers, such as low glutathione, low vitamin C levels and low SOD activity, in agreement with previous findings (Citation5,Citation23). Low plasma levels of vitamin C could reflect its high utilization rate, suggesting that this vitamin may be used to reduce oxidative stress in obese mothers. Catalase activity was normal in obese mothers while SOD activity was reduced in obese mothers compared with control values. Reduced antioxidant enzyme activities have been reported in obesity (Citation5). Antioxidant enzymes may be consumed or inactivated in high oxidative conditions.
The hypertensive mothers had relatively large and accentuated changes in the oxidant/antioxidant status. We noted increased erythrocyte MDA and protein carbonyl levels in hypertensive mothers. Oxidative stress has been proposed as an important underlying mechanism that contributes to the endothelial dysfunction associated with preeclampsia (Citation6). MDA and carbonyl proteins were found to be high in preeclampsia (Citation27). Superoxide anion levels were higher in hypertensive mothers compared with control values. Increased generation of superoxide anion, activated leukocytes and the up-regulation of NADPH oxidase were observed in preeclampsia (Citation28). In addition, NO levels were decreased in hypertensive mothers, in agreement with previous findings (Citation29). Deficiency of NO has been reported to cause preeclampsia (Citation29). Hypertensive mothers also had low glutathione, low vitamin C levels and low SOD activity, in agreement with previous findings (Citation30). Indeed, catalase activity was low in hypertensive mothers compared with control values. Reductions in SOD, the primary enzyme that inactivates the superoxide radical, and in catalase activity, which is involved in the detoxification of H2O2, would lead to increased numbers of free radicals and this could be responsible for the increased levels of superoxide anion, MDA and carbonyl proteins in hypertensive mothers.
In our study, changes in hemostasis were noted in obese and particularly hypertensive mothers. In these mothers, a procoagulant state was observed. We found an increase in clotting factors (fibrinogen, prothrombin) in both obese and hypertensive mothers, and a decrease in natural anticoagulants (protein C, protein S) in only hypertensive mothers. However, antithrombin, another anticoagulant, was increased in these hypertensive mothers.
Physiological changes during pregnancy with hypercoagulability may contribute to an increased risk of thrombosis. Multiple changes in the hemostatic system progressively occur during the course of pregnancy, with the greatest abnormalities occurring at term (Citation1,Citation2). In the organism, there is a delicate balance between bleeding and clotting, which is affected by several factors. Because natural anticoagulants are required to stop the clotting process, deficiencies in one of these substances can upset this balance and lead to thrombophilia. The most important natural anticoagulants are protein C, protein S and antithrombin.
Preeclampsia and obesity are important factors in the etiology of pregnancy-associated venous thromboembolism (Citation13,Citation14). The state of enhanced coagulation in preeclampsia was evidenced by elevated levels of total fibrinogen (Citation14). Indeed, studies have shown increased expression of pro-coagulant proteins and reduced levels of anticoagulant proteins in women with preeclampsia (Citation31). Fibrinogen levels were found to be increased in obese patients (Citation32). Excess adipose tissue contributes directly to the pro-thrombotic state. Maternal obesity is a risk factor for venous thromboembolism (Citation8).
The antiphospholipid antibody syndrome is one of the most important acquired risk factors for thrombosis. Characterized by the presence of circulating antiphospholipid antibodies or LACs in plasma, it is associated with arterial or venous thrombosis and/or pregnancy complications (Citation33). Several tests are used to detect the presence of LACs. The APTT, PTT-LA and DRVVT are common screening tests for LACs. Using these tests, we observed that obese mothers had normal results while hypertensive mothers were positive for LACs. Women with antiphospholipid syndrome (APS) and antiphospholipid antibodies are at risk of adverse pregnancy outcomes, such as preeclampsia and IUGR (Citation34). Elevated uric acid concentrations, which play a role in the induction of inflammation at the maternal–fetal interface, were noted in women with APS, leading to placental dysfunction and adverse pregnancy outcome (Citation35).
In conclusion, the combination of elevated lipid and protein oxidation markers and decreased antioxidant capacity provides a clear indication of the presence of oxidative stress in obese and hypertensive mothers. These mothers also had a pro-thrombotic state, which combined with oxidative stress could be an important contributory factor for several complications during pregnancy, especially in preeclampsia. Great importance should therefore be placed on oxidative and hemostasis markers in obese and hypertensive pregnant woman. A combination of these markers may increase the detection of abnormalities in pregnancy and allow for more effective prophylactic strategies. Obese and hypertensive mothers require closer clinical monitoring during pregnancy. The oxidative and hemostasis profile could aid in evaluating new preventive therapies before the onset of clinical symptoms or signs.
Acknowledgments
The present work was realized with the financial support of the National Agency for the Development of Health Research (PNR, ATRSS). Our thanks go to all volunteers.
Declaration of interest: None of the authors has any financial or personal conflicts of interest.
References
- James AH. Pregnancy and thrombotic risk. Crit Care Med. 2010;38:57–63.
- Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–68.
- Eichinger S, Evers JLH, Glasier A, La Vecchi, C, Martinelli I, Skouby S, et al. Venous thromboembolism in women: a specific reproductive health risk. Hum Reprod Update. 2013;19:471–82.
- Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol. 2002;57:609–13.
- Malti N, Merzouk H, Baba Ahmed FZ, Merzouk SA, Malti A, Tessier C, et al. Oxidative stress biomarkers in obese mothers and their appropriate for gestational age newborn. J Clin Diagn Res. 2010;4:2237–45.
- Siddiqui IA, Jaleel A, Tamimi W, Al Kadri HM. Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet. 2010;282:467–9.
- Sen S, Iyer C, Meydani SN. Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol. 2014;34:105–11.
- Morgan ES, Wilson E, Watkins T, Gao F, Hunt BJ. Maternal obesity and venous thromboembolism. Int J Obstet Anesth. 2012;21:253–63.
- Jim B, Sharma S, Kebede T, Acharya A. Hypertension in pregnancy. Cardiol Rev. 2010;18:178–89.
- Von Versen-Hoeynck FM, Powers RW. Maternal fetal metabolism in normal pregnancy and pre-eclampsia. Front Biosci. 2007;12:2457–70.
- Loukidi-Bouchenak B, Lamri-Senhadji MY, Merzouk S, Merzouk H, Belarbi B, Prost J, et al. Serum lecithin:cholesterol acyl transferase activity, HDL2 and HDL3 composition in hypertensive mothers and their small for gestational age newborns. Eur J Pediatr. 2008;167:525–32.
- Zhou JF, Wang XY, Shangguan XJ, Gao ZM, Zhang SM, Xiao WQ, et al. Increased oxidative stress in women with pregnancy-induced hypertension. Biomed Environ Sci. 2005;18:419–26.
- Heilmann L, Rath W, Pollow K. Hemostatic abnormalities in patients with severe preeclampsia. Clin Appl Thromb Hemost. 2007;13:285–91.
- Alwan AF, Aladdin MZ, Shaymma WS. Study of plasma fibrinogen in pregnant women with severe preeclampsia. J Dent Med Sci. 2013;8:55–9.
- Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Rad Res. 2007;41:1364–75.
- Sarig G, Brenner B. Coagulation, inflammation and pregnancy complications. Lancet. 2004;363:96–7.
- Auclair C, Voisin E. Nitroblue–tetrazolium reduction. In: Greenwald RA, editor. Handbook of methods for oxygen radical research. Boca Raton, FL: CRC Press; 1985. pp 123–32.
- Draper H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31.
- Levine RL, Garland D, Oliver CN, Climent I, Lenz AG, Ahn BW, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
- Jagota SK, Dani HM. A new calorimetric technique for the estimation of vitamin C using folin phenol reagent. Analyt Biochem. 1982;127:178–82.
- Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–968.
- Ophir E, Dourleshter G, Hirsh Y, Fait V, German L, Bornstein J. Newborns of pre-eclamptic women: a biochemical difference present in utero. Acta Obstet Gynecol Scand. 2006;85:1172–8.
- Malti N, Merzouk H, Merzouk SA, Loukidi B, Karaouzene N, Malti A, et al. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta. 2014;35:411–6.
- Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–9.
- Keaney JF, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–9.
- Ghasemi A, Zahediasl S, Azizi F. Elevated nitric oxide metabolites are associated with obesity in women. Arch Iran Med. 2013;16:521–5.
- Rani NA, Naidu JN. Protein carbonylation, lipid peroxidation and serum alpha tocopherol activity in preeclampsia. Int J Recent Trends Sci Technol. 2013;8:163–6.
- Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in preeclampsia. J Hypertens. 2003;21:395–402.
- Sahu S, Daniel M, Abraham R, Vedavalli R, Senthilvel V. Study of uric acid and nitric oxide concentrations in preeclampsia and normal pregnancy. Int J Biol Med Res. 2011;2:390–3.
- Suhail M, Suhail MF, Khan H. Role of vitamins C and E in regulating antioxidant and pro-oxidant markers in preeclampsia. J Clin Biochem Nutr. 2008;43:210–20.
- Paternoster D, Stella A, Simioni P, Trovo S, Plebani P, Girolami A. Clotting inhibitors and fibronectin as potential markers in preeclampsia. Int J Gynecol Obstet. 1994;47:215–21.
- Faber DR, De Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009;10:554–63.
- Urbanus RT, Derksen RHMW, De Groot PG. Current insight into diagnostics and pathophysiology of the antiphospholipid syndrome. Blood Rev. 2008;22:93–105.
- Tincani A, Bazzani C, Zingarelli S, Lojacono A. Lupus and the antiphospholipid syndrome in pregnancy and obstetrics: clinical characteristics, diagnosis, pathogenesis, and treatment. Semin Thromb Hemost. 2008;34:267–73.
- Mulla MJ, Salmon JE, Larry W, Brosens JJ, Boeras CM, Kavathas PB, et al. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1b production by human first trimester trophoblast. PLoS One 2013;8:e65237.
