Abstract
Takayasu's arteritis (TA) is a chronic, idiopathic, inflammatory disease affecting the aorta and its branches. To date, only one case involving abdominal aortic thrombosis due to TA has been reported. After bilateral artificial subclavian–iliac bypass, a case of abdominal aortic thrombosis due to TA received a delayed diagnosis in a 44-year-old Chinese male who experienced recurrent episodes of heart failure and uncontrolled hypertension with claudication of two extremities. Abdominal color Doppler sonography and computed tomography aortography (CTA) showed occlusion of the abdominal aorta and bilateral renal artery stenosis. After vascular bypass and during 1 year follow-up, his cardiac function improved and blood pressure was well controlled, with reduced serum creatinine. Postoperative CTA still showed abdominal aortic thrombosis resulting in arterial occlusion extending from the left renal artery initial segment level to the bilateral common iliac artery and the bifurcation of the renal artery, except for the vascular bypass. Abdominal aortic thrombosis due to TA is very rare and potentially life threatening, probably becoming an atherosclerosis risk factor. Doppler sonography and CTA results are important for diagnosis. Artificial vascular bypass can be used for TA in debilitated patients with diffuse aortic disease.
Introduction
Takayasu's arteritis (TA) is a chronic, idiopathic, inflammatory disease that primarily affects large vessels, such as the aorta and its major branches. Historically, Mikito Takayasu, a Japanese ophthalmologist, described a peculiar wreath-like arteriovenous anastomosis around the papillae of the retina (Takayasu disease) in 1908 (Citation1). In the first necropsy case reported in 1940, this ophthalmological finding was related to cervical vessel occlusion (Citation2,Citation3). Subsequently, this non-specific panarteritis that affects the intima and the adventitia of the aorta and its main branches was called TA. Its clinical manifestations are varied and relate to the vessel that presents the stenotic or occlusive lesions, such as the aortic arch [pulseless disease (Citation4)], descending thoracic or abdominal aorta [atypical coarctation (Citation5)], renal arteries (Citation6), coronary arteries (Citation7) and pulmonary arteries (Citation8). The most common site of stenosis is the subclavian and innominate arteries. Renal artery involvement in TA patients is common, especially in Asia, explaining the elevated incidence of hypertension (Citation9,Citation10). Cardiac, renal and central nervous system involvement are the principal causes of severe morbidity and mortality. Since large-artery biopsies cannot easily be done in patients with suspected TA, the non-specific clinical presentations and laboratory test results frequently contribute to late diagnosis and delayed treatment (Citation11). Heart failure due to abdominal aortic thrombosis is a rare clinical manifestation of TA; so far, only one case of this kind has been reported (Citation12).
In this report, we present the case of an adult man of TA with recurrent episodes of heart failure and uncontrolled hypertension due to abdominal aortic thrombosis diagnosis being delayed after bilateral artificial subclavian–iliac bypass. We review the diagnosis and treatment of TA with abdominal aortic thrombosis.
Case report
A 44-year-old Chinese man was hospitalized at our hospital for further examinations of recurrent episodes of heart failure and uncontrolled hypertension with claudication of two extremities with a duration of nearly 4 years. The heart failure first developed more than 3 years ago with sudden dyspnea and palpitation, and had relapsed three times over the past 2 months. Medical history included hypertension and hyperglycemia for nearly 4 years, managed with candesartan (4 mg/day), amlodipine (10 mg/day) and sustained-release metformin hydrochloride (2 g/day). Hypertension was not controlled with the above combination of antihypertensive drugs. He had smoked 30 cigarettes a day for 30 years.
On physical examination, he had a regular pulse of 65 beats/min. His blood pressure (BP) in the upper limbs was 192/104 mmHg (right) and 192/104 mmHg (left), while the BP in the lower limbs was 130/91 mmHg (right) and 125/86 mmHg (left). There was a 3/6 systolic murmur over the abdominal umbilical area. BP was measured in the sitting position using a standardized aneroid sphygmomanometer. The patient had been resting for 30 min before the measurement. Pulses of the bilateral radial arteries, along with the right dorsalis pedis artery, were symmetrical and could be touched. The bilateral femoral artery, popliteal artery and the left dorsalis pedis artery could not be touched. Twenty-four-hour ambulatory blood pressure monitoring (ABPM) measurements were performed with a Holter & ABPM Combiner CB-1804-B (China Manufacturer, Trading Company of BIOX Instruments Co.). The system takes three measurements per hour during daytime and one measurement per hour during night-time. Normal 24 h ambulatory BP is defined as 130/80 mmHg. Concurrently, normal daytime and night-time BP levels are defined as 135/85 mmHg and 120/70 mmHg, respectively. ABPM revealed an average BP of 159/86 mmHg, average daytime BP of 161/87 mmHg and average night-time BP of 153/81 mmHg with a non-dipping pattern.
A 12-lead electrocardiogram revealed a normal sinus rhythm, left atrial overloading and myocardial ischemia (). A chest X-ray showed cardiomegaly (cardiothoracic ratio 57.4%) (). Echocardiography showed left ventricle hypertrophy (left ventricular mass index 188.30 g/m2, normal man < 125 g/m2). Myocardial perfusion imaging revealed left ventricle hypertrophy (left ventricular ejection fractions 35%, normal > 50%). Color Doppler ultrasound showed an echo filling at the renal artery level of the abdominal aorta, and superior mesenteric artery with widened diameter and increased velocities (). Thickening of the intima media at the bilateral renal artery opening was found, with significantly reduced diameter (). Bilateral iliac artery collateral circulation was observed. Computed tomography aortography (CTA) revealed occlusion of the abdominal aorta and bilateral renal artery stenosis. Multiple collateral channels were seen in the bilateral renal artery, mesenteric artery and bilateral iliac artery, accompanied by transmural calcification (see ). Laboratory values were as follows: urine protein 2+ (normal –), 2 h postprandial blood glucose 12 mmol/l (normal < 11.1 mmol/l), serum creatinine 247 μmol/l (normal < 133 μmol/l), serum potassium 4.5 mmol/l (normal 3.5–4.5 mmol/l) and prohormone brain natriuretic peptide (proBNP) 4512 pg/ml (normal < 450 pg/ml). The results of other tests, including fasting plasma glucose, hemoglobin A1c, serum troponin I, antistreptolysin O, C-reactive protein, erythrocyte sedimentation rate, autoimmunity, humoral immunity, disseminated intravascular coagulation, coagulation profile, thyroid function, plasma renin activity, plasma angiotensin II, plasma aldosterone concentration, 24 h urine vanillylmandelic acid and 24 h blood catecholamine, were within normal ranges.
Figure 1. (A) Twelve-lead electrocardiogram showing sinus rhythm, PTFV1 ≥ 0.04 mm s, TavL flat, TavF inversion, TV4–6 bidirectional, STV5–6 depression. (B) Chest X-ray showing cardiomegaly (cardiothoracic ratio 57.4%).
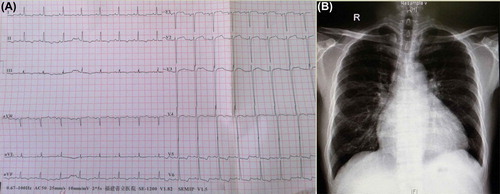
Figure 2. (A) Abdominal color Doppler ultrasonography showing an echo filling (thin arrow) at the double renal artery level of the abdominal aorta and superior mesenteric artery, widened diameter (thick arrow) with increased velocities (PS 208 cm/s). (B) The intima media thickness at bilateral renal artery opening (arrows) was found; the diameters of the arteries were significantly reduced.
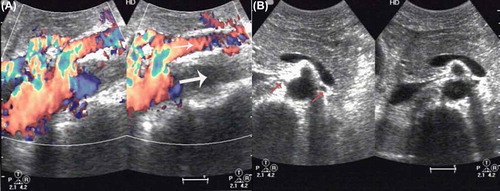
Figure 3. Bedside chest X-ray showing acute pulmonary edema: enlarged heart size and apical vascular redistribution and small bilateral pleural effusions.
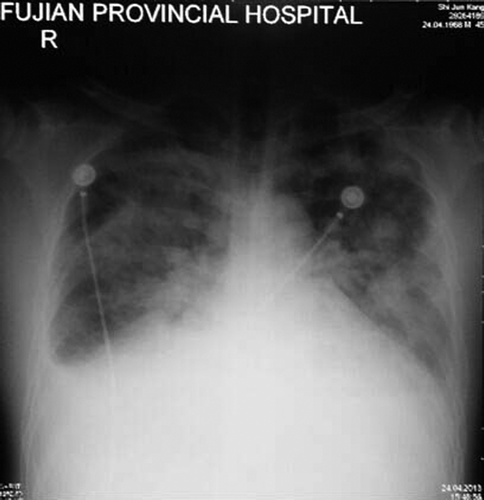
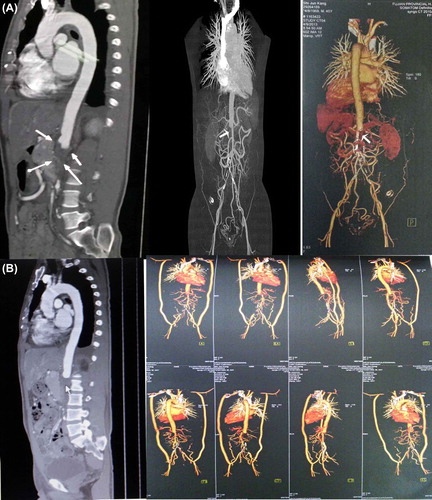
Figure 4. (A) After review of the case: computed tomography aortography (CTA) showing abdominal aortic thrombosis (arrows) that resulted in arterial occlusion extending from the left renal artery initial segment level to the bilateral common iliac artery and the bifurcation of the renal artery. Multiple collateral channels were seen in the bilateral renal artery, mesenteric artery and bilateral iliac artery, accompanied by transmural calcification. (B) After bilateral artificial subclavian–iliac bypass: CTA showing abdominal aortic thrombosis (arrow) that resulted in arterial occlusion extending from the left renal artery initial segment level to the bilateral common iliac artery and the bifurcation of the renal artery, except for the vascular bypass.
A diagnosis of abdominal aorta stenosis with bilateral renal artery stenosis was made. After furosemide 20 mg/day, amlodipine 5 mg/day, valsartan 80 mg/day, metformin 1 g*2/day treatment, the patient's BP was controlled within 100–145/54–68 mmHg, proBNP dropped to 2183 pg/ml, serum creatinine to 153 μmol/l and serum potassium to 3.9 mmol/l. He was scheduled for a vascular bypass operation. During the preoperative coronary CTA test, he had sudden dyspnea and coughing of pink frothy sputum. His proBNP increased to 20,884.00 pg/ml with serum creatinine 278 μmol/l, and a bedside chest X-ray showed acute pulmonary edema (). After treatment including continuous renal replacement therapy, his condition stabilized and proBNP dropped to 1210.00 pg/ml with serum creatinine 166 μmol/l. He then underwent a bilateral artificial subclavian–iliac bypass operation. His postoperative BP was well controlled and the pulses of bilateral dorsalis pedis arteries were symmetrical and strong.
Postoperation serum creatinine tests were between 179 and 195μmol/l. The patient was discharged 11 days after the operation. During 1 year follow-up, his cardiovascular condition was acceptable and his BP range was 150–130/90–80 mmHg, with reduced serum creatinine under medication including antihypertensive (amlodipin 10 mg/day and candesartan 4 mg/day), antidiabetic (metformin hydrochloride sustained release 2 g/day), antiplatelet (aspirin 100 mg/day) and statin therapy (atorvastatin 20 mg/day). After review of this case, CTA showed abdominal aortic thrombosis (), resulting in arterial occlusion extending from the left renal artery initial segment level to the bilateral common iliac artery and the bifurcation of the renal artery, except for the vascular bypass ().
Discussion
In reference to the American College of Rheumatology criteria in 1990 (Citation13), this patient had four out of the six criteria for TA (onset at age ≤ 40 years, claudication of two extremities, a bruit over the abdominal aorta, and arteriographic evidence of occlusion and narrowing of the abdominal aorta and the renal arteries). According to Sharma's criteria for the diagnosis of TA (Citation14), this patient met one major criterion (characteristic signs and symptoms of at least 1 month's duration including limb claudication, a significant blood presence difference and dyspnea) and two minor criteria (hypertension and abdominal aorta lesion). Hence, the diagnosis of TA could be made. According to the vessels involved, the angiographic classification divides TA into six types (Citation15). Type I involves only the branches of the aortic arch. Type IIa involves the ascending aorta, aortic arch and its branches. Type IIb affects the ascending aorta, aortic arch and its branches, and thoracic descending aorta. Type III involves the descending thoracic aorta, abdominal aorta and/or renal arteries. The ascending aorta, aortic arch and its branches are not affected. Type IV involves only the abdominal aorta and/or renal arteries. Type V has combined features of types IIb and IV. Based on his CTA results, this patient's aortic disease belongs to type IV TA.
TA usually affects young or middle-aged females; however, recently male or elderly patients with this disease have been reported (Citation8,Citation16). Our patient was a middle-aged male and his disease development could be divided into two phases. In the early pre-pulseless phase, patients may complain of systemic symptoms including fever, weight loss, malaise, headaches, carotidynia, myalgia and arthralgia. In the later pulseless phase, commonly appearing months to years later, symptoms reflect end-organ ischemia and include limb claudication and neurological symptoms. The non-specific nature of presenting symptoms and the lack of specific laboratory markers often delay the diagnosis, with potentially life-threatening consequences (Citation17). For our patient, TA was not taken into consideration until recurrent episodes of heart failure and uncontrolled hypertension, with a significant difference between upper limb BP and lower limb BP. Abdominal color Doppler sonography and CTA (Citation18) confirmed the occlusion of the abdominal aorta and bilateral renal artery stenosis. Moreover, although diagnostic criteria exist, patients presenting with early prestenotic disease may not fulfill them (Citation19). Compared with abdominal occlusion, ascending aortic aneurysms occurred more frequently in males with TA, but all they had a more severe disease profile in males (Citation20). Subramanyan et al. regarded the presence of severe functional disability and cardiac involvement as predictors of either death or major events on follow-up (Citation21). If TA involves the descending thoracic aorta and the abdominal aorta, patient can present with heart failure due to severe aortic and/or abdominal aortic stenosis (Citation22–24) or accompanied by atherosclerosis (Citation25).
Vessel wall inflammation, atherosclerosis and hypercoagulability may be responsible for ischemic events in TA. Our patient had atherosclerotic risk factors such as smoking, hypertension and diabetes mellitus. In TA, the coexistence of arteritis and atherosclerosis has been reported and some of these cases show remarkable atherosclerotic lesions in the coronary arteries as well as in other vessels, such as atherosclerotic plaques (Citation26), aortic and coronary calcifications (Citation27). A hypercoagulable state has been described in TA patients that makes thrombosis a concern in the affected vessels, with stenotic/occlusive lesions or even aneurysms, and may result in acute ischemic events (Citation28). TA patients present higher levels of platelet P selectin (Citation29) and plasma thromboxane B2 (Citation30) and lower levels of 6-keto-prostaglandin F1α, especially in those with an active phase who have enhanced platelet aggregation in response to collagen (Citation31). Inflammation, accelerated atherosclerosis and modification of laminar blood flow due to distortion of the vessel wall anatomy may all contribute to the occurrence of arterial thrombosis and ischemic events in TA patients. Inflammation increases procoagulant factors, and also inhibits natural anticoagulant pathways and fibrinolytic activity, causing a thrombotic tendency. Chronic inflammation may cause endothelial damage, resulting in the loss of physiological anticoagulant, antiaggregant and vasodilatory properties of the endothelium (Citation32); therefore, inflammation can induce thrombosis. Asai et al. reported a patient with a painful mass on the right lateral neck, which was diagnosed as TA according to ultrasonographic findings and clinical implications (Citation33). They found a calcified thrombus at the bulb leading to total obstruction of the right common carotid artery and a substantial increase in the intima–media thickness of the adjacent artery walls. In our patient, the formation of a thrombus obstructing the abdominal aortic artery was so slow that there was enough time for collateral vessels to develop and thus to supply sufficient blood to the abdominal organs, as revealed by Doppler ultrasonography and CTA. In a study by Chung et al., CTA was performed from the carotid bifurcation to the iliac bifurcation in 85 consecutive patients with TA (Citation34). They found that aortic involvement in TA could occur from the aortic root to below the iliac bifurcation. Aortic involvement occurs in a contiguous, synchronous fashion in most patients. Delayed contrast- enhanced magnetic resonance imaging could reveal diffuse enhancement of the aortic wall with direct histological evidence of inflammation in a patient with active TA but normal inflammatory markers (Citation35). Arnaud et al. studied peripheral vascular Doppler, CTA and angiomagnetic resonance imaging data (Citation36). They revealed that TA lesions mostly develop in a symmetrical manner in paired vascular territories and are contiguous in the aorta. Our patient suffered from intima media thickness at the bilateral renal artery opening. This may prove useful for improving the vascular Doppler and radiological follow-up of patients with TA (Citation37), since those who have involvement of a given paired artery are expected to develop new lesions more often in the contralateral territory than in any other territory. Careful monitoring of the contralateral artery may be useful to detect early involvement such as vascular wall thickening, and thus may result in an early and more effective treatment modification (Citation38,Citation39).
Early initiation of immunosuppressive treatment is crucial to control active inflammation and minimize arterial injury in patients with TA. Poor outcomes are attributed to a delay in diagnosis, in part due to a lack of awareness of the condition, late administration of medical treatment, and inappropriate patient selection and timing of vascular intervention (Citation19). In this case, the late diagnosis of TA and lack of early immunosuppressive treatment led to his abdominal aortic thrombosis, recurrent episodes of heart failure and uncontrolled hypertension. By the time he was hospitalized, his immunological indicators were within normal ranges, meaning that his inflammation had become chronic and there was no indication for immunosuppressive treatment. Ishikawa defined the four most important complications of TA as retinopathy, secondary hypertension, aortic regurgitation and aneurysm formation, each graded as mild, moderate or severe (Citation40). The disease commonly presents in the second or third decade of life, with a delay in diagnosis of between months and years from the onset of first symptoms, as was the case in our patient. With symptomatic stenotic or occlusive lesions, it appears appropriate and often necessary to revascularize. The literature, however, indicates that these procedures should be carefully considered and that restenosis is common; therefore, intervention should be reserved for specific indications. The indications for considering intervention include uncontrolled hypertension due to renal artery stenosis, severe symptomatic coronary artery or cerebrovascular disease, severe aortic regurgitation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysms at risk of rupture (Citation41). In these cases, the risk/benefit ratio for intervention is good. Open surgery, at present, has better outcomes than endovascular techniques (Citation42). Biological inflammation increases the likelihood of complications after revascularization in patients with TA (Citation43).
Axillofemoral bypass is a widely accepted management approach that carries a low risk of mortality and morbidity. The results from our patient indicate that bilateral artificial subclavian–iliac bypass reduces the hemodynamic disturbances. This benefit appears to be the result of the reduction of cardiac afterload and preservation of renal and mesenteric perfusion. Therefore, bypass grafting is a viable alternative to direct aortic reconstruction in the treatment of patients with severe cardiac disease, with highly reproducible 3 year and 5 year patency rates (Citation44). Although aortofemoral bypass remains the procedure of choice in low-risk patients, axillofemoral or subclavian–iliac grafting remains an excellent therapeutic option for surgeons encountering elderly or debilitated patients with diffuse aortic disease. There is no doubt that we should treat atherosclerosis risk factors in patient with TA. Ueno pointed out that first line medical treatment for TA is the use of corticosteroids as anti-inflammatory agents. Adjunctive antiplatelet or antihypertensive agents are recommended as second line medical treatment in patients with TA (Citation45). A study by de Souza et al. indicated that antiplatelet therapy with aspirin dosage between 100 and 200 mg/day reduces the risk of acute ischemic events (Citation46). Our patient was followed up for 1 year after surgery. His BP and serum creatinine were stable under antihypertensive, antidiabetic, antiplatelet and statin therapies.
In conclusion, abdominal aortic thrombosis due to TA is very rare and potentially life threatening, probably becoming an atherosclerosis risk factor. Doppler sonography and CTA are important in early diagnosis and treatment modification. Artificial vascular bypass can be used for TA in debilitated patients with diffuse aortic disease.
Funding: This work was supported by the Natural Science Foundation of Fujian Province in China [No. 2014Y0012].
Declaration of interest: The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Takayasu M. A case with peculiar changes of the central retinal vessels. Acta Soc Opthalmol Jpn. 1908;12:554–5. (In Japanese.)
- Numano F, Kobayashi Y. Takayasu arteritis: beyond pulselessness. Intern Med. 1999;38:226–32.
- Numano F, Okawara M, Inomata H, Kobayashi Y. Takayasu's arteritis. Lancet. 2000;356:1023–5.
- Shimizu K, Sano K. Pulseless disease. Clin Surg (Tokyo). 1948;3:377–96.
- Inada K, Yokoyama T, Nakata R. Atypical coarctation of the aorta. Angiology. 1963;14:506–17.
- Utz G, Simon B, Mickisch R, Dönert G, Zebe H. Giant-cell (Takayasu) arteritis as a cause of renovascular hypertension. Z Kardiol. 1975;64:482–8.
- Cipriano PR, Silverman JF, Perlroth MG, Griepp RB, Wexler L. Coronary arterial narrowing in Takayasu's aortitis. Am J Cardiol. 1977;39:744–50.
- Fukuda Y, Shirai K, Takamiya Y, Nathan M, Mito T, Yamagi D, et al. Isolated pulmonary arterial stenosis caused by Takayasu's arteritis in an elderly male. J Cardiol. 2008;51:196–200.
- Zhu G, He F, Gu Y, Yu H, Chen B, Hu Z, et al. Angioplasty for pediatric renovascular hypertension: a 13-year experience. Diagn Interv Radiol. 2014;20:285–92.
- Sadurska E, Jawniak R, Majewski M, Czekajska-Chehab E. Takayasu arteritis as a cause of arterial hypertension. Case report and literature review. Eur J Pediatr. 2012;171:863–9.
- Mason JC. Takayasu arteritis – advances in diagnosis and management. Nat Rev Rheumatol. 2010;6:406–15.
- Chaudhary SC, Gupta A, Himanshu D, Verma SP, Khanna R, Gupta DK. Abdominal angina: an unusual presentation of Takayasu's arteritis. BMJ Case Rep. 2011;2011:bcr0220113900.
- Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34.
- Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54(Suppl):S141–7.
- Zhu FP, Luo S, Wang ZJ, Jin ZY, Zhang LJ, Lu GM. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol. 2012;85:e1282–92.
- De Caridi G, Butrico L, Grande R, Massara M, Spinelli F, de Franciscis S, et al. Concomitant aortic leiomyosarcoma and Takayasu arteritis in a 55-year-old male patient. Ann Vasc Surg. 2014;28:1931.e13–6.
- Nazareth R, Mason JC. Takayasu arteritis: severe consequences of delayed diagnosis. QJM. 2011;104:797–800.
- Lambert M, Hatron PY, Hachulla E, Warembourg H, Devulder B. Takayasu's arteritis diagnosed at the early systemic phase: diagnosis with noninvasive investigation despite normal findings on angiography. J Rheumatol. 1998;25:376–7.
- Mason JC. Takayasu arteritis – advances in diagnosis and management. Nat Rev Rheumatol. 2010;6:406–15.
- Mont’Alverne AR, Paula LE, Shinjo SK. Features of the onset of Takayasu's arteritis according to gender. Arq Bras Cardiol. 2013;101:359–63.
- Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu's disease) Circulation. 1989;80:429–37.
- Yang MC, Yang CC, Chen CA, Wang JK. Takayasu arteritis presenting with acute heart failure. J Am Coll Cardiol. 2013;61:1302.
- Liu GR, Guo X, Huang LJ. Heart failure caused by Takayasu arteritis. Intern Med. 2014;53:811–2.
- Murayama A, Inagaki T, Watanabe K, Umebayashi H, Miura K, Abukawa D, et al. Acute heart failure due to midaortic occlusion as the initial manifestation of Takayasu arteritis. Circ Heart Fail. 2009;2:74–6.
- Okada H, Suzuki H, Murakami M, Ogata Y, Takenaka T, Sakaguchi H, et al. Takayasu's arteritis with heart failure due to atherosclerosis. Jpn J Med. 1990;29:309–12.
- Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S, et al. Atherosclerosis in Takayasu arteritis. Ann Rheum Dis. 2006;65:1202–7.
- Seyahi E, Ucgul A, Cebi Olgun D, Ugurlu S, Akman C, Tutar O, et al. Aortic and coronary calcifications in Takayasu arteritis. Semin Arthritis Rheum. 2013;43:96–104.
- Akazawa H, Ikeda U, Yamamoto K, Kuroda T, Shimada K. Hypercoagulable state in patients with Takayasu's arteritis. Thromb Haemost. 1996;75:712–6.
- Kasuya N, Kishi Y, Isobe M, Yoshida M, Numano F. P-selectin expression, but not GPIIb/IIIa activation, is enhanced in the inflammatory stage of Takayasu's arteritis. Circ J. 2006;70:600–4.
- Numano F, Shimokado K, Kishi Y, Nishiyama K, Türkoglu C, Yajima M, et al. Changes in the plasma levels of thromboxane B2 and cyclic nucleotides in patients with Takayasu disease. Jpn Circ J. 1982;46:16–20.
- Watanabe T, Kishi Y, Numano F, Isobe M. Enhanced platelet sensitivity to prostacyclin in patients in an active stage of Takayasu arteritis. Thromb Res. 2001;104:77–83.
- Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18:1478–93.
- Asai S, Matsushita H, Komiya S, Yamamoto S, Miyachi H. Ultrasonographic diagnosis of a cervical mass caused by a calcified thrombus as an initial manifestation of Takayasu arteritis. J Ultrasound Med. 2007;26:271–4.
- Chung JW, Kim HC, Choi YH, Kim SJ, Lee W, Park JH. Patterns of aortic involvement in Takayasu arteritis and its clinical implications: evaluation with spiral computed tomography angiography. J Vasc Surg. 2007;45:906–14.
- Ginde S, Cava JR, Southern JF, Saudek DE. Delayed contrast- enhanced magnetic resonance imaging in the evaluation of Takayasu arteritis. J Am Coll Cardiol. 2012;59(12):e23.
- Arnaud L, Haroche J, Toledano D, Cacoub P, Mathian A, Costedoat-Chalumeau N, et al. Cluster analysis of arterial involvement in Takayasu arteritis reveals symmetric extension of the lesions in paired arterial beds. Arthritis Rheum. 2011;63:1136–40.
- Liu Y-g, Zhang X-d. The study of color Doppler sonography and CT angiography in diagnosis of Takayasu arteritis. J Med Imaging. 2013;23:1095–7.
- Earl Schmidt WA. Role of ultrasound in the understanding and management of vasculitis. Ther Adv Musculoskelet Dis. 2014;6:39–47.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford). 2014; 53:793–801.
- Ishikawa K. Natural history and classification of occlusive thromboaortopathy (Takayasu's disease). Circulation. 1978;57:27–35.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med. 2013;2013:618910.
- Kim YW, Kim DI, Park YJ, Yang SS, Lee GY, Kim DK, et al. Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg. 2012;55:693–700.
- Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, et al. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. 2012;125:813–9.
- Bang JH, Kim JW, Jung SH, Lim JY. Axillofemoral bypass to treat severe heart failure caused by Takayasu's arteritis. Korean J Thorac Cardiovasc Surg. 2012;45:124–6.
- Ueno M. Antiplatelet therapy in the treatment of Takayasu arteritis. Circ J. 2010;74:1079–80.
- de Souza AW, Machado NP, Pereira VM, Arraes AE, Reis Neto ET, Mariz HA, Sato EI. Antiplatelet therapy for the prevention of arterial ischemic events in Takayasu arteritis. Circ J. 2010;74:1236–41.
