Abstract
The purpose of this study was to assess the vasoconstrictive effects of adenosine in the kidney microcirculation in hypertensive patients with renal artery stenosis (RAS). Twelve patients with resistant hypertension and moderate RAS were selected for the study. In all patients, systolic, diastolic and mean translesional pressure gradients, distal pressure (Pd), aortic pressure (Pa) and Pd/Pa ratio were measured using a pressure guidewire at baseline and after intrarenal bolus administration of 400 μg adenosine. We observed significant changes in mean translesional pressure gradient and systolic Pd after pharmacological stimulation. The results suggest that in hypertensive patients with RAS, vasomotor activity of the kidney microcirculation may be preserved.
Introduction
The efficacy of renal artery stenting in treating arterial hypertension caused by renal artery stenosis (RAS) remains an issue of debate. A number of studies (Citation1–3) showed poor correlations between stenotic renal artery diameter by quantitative angiography and hemodynamic significance of the lesion. Nevertheless, in all studies, angiography was selected as the primary decision-making criterion for performing renal artery stenting. This may be one of the main reasons for the unsatisfactory results obtained with renal angioplasty.
Recently, it was postulated that translesional pressure gradient measurements and hyperemic renal fractional flow reserve (FFR) may be promising markers of hypertensive patients with RAS who may benefit from renal artery stenting in terms of blood pressure control (Citation3,Citation4). In these studies, a number of diverse pharmacological agents (dopamine, papaverine and fenoldopam) was used to assess the vasodilatation of the renal microcirculation. To date, a limited number of studies has analyzed the ability of the renal microcirculation to contract in response to pharmacological agents. Moreover, the results of these observations are confounding (Citation5–7). The afferent arteriolar vasoconstriction may be important for renal parenchyma protection in the early post-angioplasty period, when the hypotensive response to revascularization may not yet be noticeable but the damaging effects of hypertension are still present. In theory, this temporal post-angioplasty vasoconstriction may be especially beneficial not for pressure control but for glomerular protection and proper long-term renal filtration function.
The objective of the present study was to assess the existence and magnitude of the renal microcirculation response to adenosine as a potential vasoconstricting agent, which could be used in future studies on the effects of RAS revascularization.
Methods
Twelve patients (50% male, age 65 ± 10.4 years) with resistant hypertension and moderate unilateral RAS (50–70%) with normal glycemic control were selected for the present study. All individuals, who were candidates for renal revascularization, signed an informed consent form. The study protocol was approved by the local ethics committee. Resistant hypertension was diagnosed by randomly measured values of arterial blood pressure exceeding the guideline values (> 140/90 mmHg), despite the use of three hypotensive medications, including a diuretic. Baseline characteristics of the study population are shown in .
Table I. Baseline clinical and procedural characteristics.
In all patients, the procedure was performed using Judkin's technique. In brief, after the introduction of a 6 F sheath into the femoral artery, a 6 F right coronary guiding catheter was advanced to the ostium of the renal artery. An intrarenal bolus of 400 μg isosorbite dinitrate was administered before angiography to avoid interlobar arterial spasm and its confounding effect on hemodynamic measurements. Angiographic images of the renal artery were analyzed offline by a skilled analyst using a digital image analysis system (Innova 2000; General Electric, New York, USA). Once the baseline angiographic images had been obtained, a pressure-sensing wire (PressureWire Certus; RADI, Uppsala, Sweden) was advanced into the prestenotic section of the renal artery and calibrated. After equalization, the PressureWire catheter was placed distal to the stenosis to assess distal pressure (Pd). Aortic pressure (Pa) was measured through the guiding catheter, after its disengagement from the artery ostium to prevent pressure damping. Systolic, diastolic and mean pressure gradients (SG, DG and MG, respectively) and Pd/Pa ratio were analyzed at baseline and after pharmacological stimulation of the microcirculation.
Renal microcirculation vasoconstriction was achieved by the direct intrarenal injection of 400 μg adenosine. Because of its short-lived mode of action and very rapid degradation, the adenosine translesional SG, DG, MD and Pd/Pa were measured immediately after administration. After these parameters had returned to baseline values, control angiography was performed. To exclude effects of the administered drug on hemodynamic measurements, the heart rate and systemic pressure were continuously monitored.
Statistics
Statistical analysis was performed using R for Windows, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and MedCalc for Windows, version 12.3 (MedCalc Software, Mariakerke, Belgium). Arithmetic means and standard deviations of quantitative variables were acquired. The distribution of the data was assessed using the D’Agostino–Pearson test. For quantitative dependent variables with a normal distribution, univariate parametric analysis of variance (ANOVA) was applied. In cases of non-normal distributions for quantitative dependent variables, a non-parametric Friedman's ANOVA was used. Statistical differences between arithmetic means were evaluated by post-hoc tests. Results for the qualitative variables were expressed as percentages (%). Results were considered statistically significant when a p value was less than 0.05.
Results
In all cases, the procedure of hemodynamic measurement was successful, without any complications.
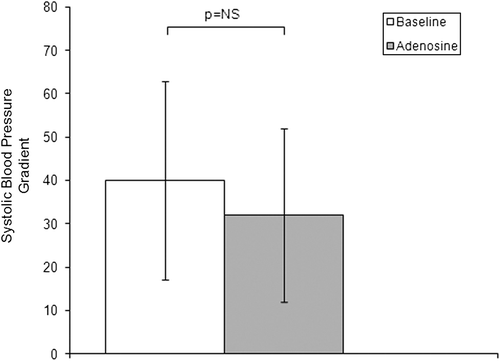
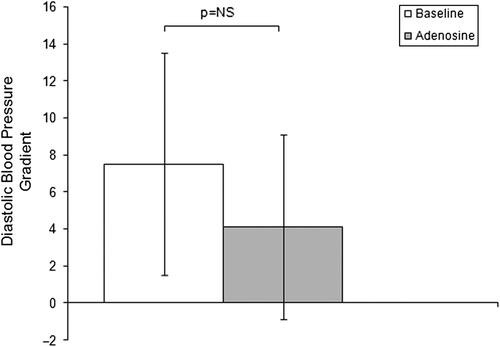
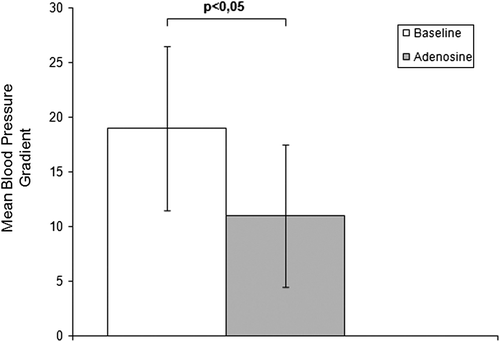
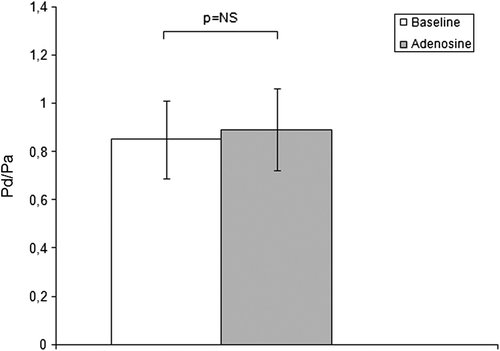
The administration of an intrarenal bolus of adenosine resulted in a significant increase in systolic Pd. There were no significant changes in diastolic Pd, mead Pd or any Pa values during the entire study. The results of pressure measurements are presented in . Translesional gradients, as well as Pd/Pa measurements at baseline and after pharmacological stimulation, are shown in . Values of MG after stimulation with 400 μg adenosine dropped significantly compared to baseline conditions. No significant differences in SG and DG were observed. Despite the influence on MG after administration of the pharmacological agent, the mean renal arterial diameter did not change (5.0 ± 0.7 mm at baseline vs 5.0 ± 0.6 mm after adenosine; p value not significant). Systemic blood pressure (161 ± 29 mmHg at baseline vs 157 ± 27 mmHg after adenosine; p not significant) and heart rate (78 ± 12 bpm at baseline vs 82 ± 14 bpm after adenosine; p not significant) were stable during the whole procedure in all individuals.
Table II. Blood pressure measurements.
Discussion
To best of our knowledge, the present study is one of the first analyses to establish the renal microcirculation response to a vasoconstrictive pharmacological agent in hypertensive patients with RAS.
In most blood vessels, adenosine provokes marked vasodilatation, which is mediated by A2a and A2b adenosine receptors. In contrast, A1 adenosine receptors, which are mainly present in renal afferent arterioles, close to and inside the glomerulus, constrict smooth muscle cells and are the primary mediators of renal blood flow autoregulation (Citation5). Adenosine produced by renal tissue has a potent effect on renal blood flow distribution and glomerular filtration rate (Citation8). Previous evidence has shown that bolus injections of adenosine cause immediate, but transient, afferent arteriole constriction and reduction in renal blood flow, reflecting the response to activation of A1 receptors (Citation9–11). Adenosine A2 receptor stimulation leads to vasodilatation and enhanced perfusion of the medulla.
The results noted in the present study are in accordance with most previous human and animal observations. Marraccini et al. (Citation6) showed a transient reduction in renal blood flow velocity with a peak systolic flow velocity decrease of approximately 60% after an intrarenal bolus injection of adenosine in humans with no RAS. In neonatal hamster kidney, adenosine applied through micropipettes caused a dose-dependent vasoconstriction of afferent arterioles (Citation12). In rabbit isolated afferent arterioles, the addition of adenosine to the bath caused a dose-dependent vasoconstriction (Citation13). Only Wierema et al. described adenosine-induced renal microcirculation vasodilatation in patients with RAS (Citation7).
The vasodilatory response of kidneys has been proposed to be an aid in identifying patients with hemodynamically significant RAS who will benefit from revascularization. However, very little is known about the role of microvascular vasoconstriction as a potential predictor of preserved renal filtration function. The ability to induce a hyperemic state suggests a persistence of viable renal parenchyma without significant ischemic tissue damage. Leesar et al. (Citation14) showed that a papaverine-mediated hyperemic systolic translesional gradient greater than 21 mmHg determined which patients benefitted from renal artery stenting in terms of blood pressure control. Mangiacapra et al. (Citation4) showed that a dopamine -induced mean translesional gradient greater than 20 mmHg is highly predictive of arterial hypertension improvement after renal stenting. In our study, mean baseline and mean hyperemic translesional gradients were significantly correlated with a decrease in arterial blood pressure after renal revascularization (Citation15).
The main purpose of the present study was to investigate the potential of adenosine to trigger vasoconstriction of the kidney microcirculation in patients with RAS, not to establish the role of the microcirculation constriction in predicting the effects of revascularization on renal function. Nevertheless, the influence of adenosine on translesional MG may be of relevant importance and should be assessed in further studies. Despite the fact that adenosine stimulation did not result in significant changes in SG, DG or Pd/Pa, changes in MG suggest the preserved potential of renal microcirculation to vasoconstriction. Loutzenhiser et al. presented the results of several studies (Citation16,Citation17) suggesting that afferent arteriolar vasoconstriction is the primary mechanism protecting the glomerulus from the damaging effects of hypertension. According to their observations, the transmission of elevated blood pressure to the glomerulus induces glomerular injury and plays a crucial role in the pathogenesis of chronic kidney disease. In addition, Hartman (Citation18) suggests that afferent arteriole spasm arises as a consequence of hypertension. By repairing the stenosis and restoring proper blood flow after renal revascularization, we eliminate the reason for kidney ischemic damage, as shown by Goldblatt et al. (Citation19); however, according to the Loutzenhiser hypothesis, the inability of the afferent arteriole to appropriately adjust its contraction in response to the elevated systolic blood pressure increases the risk of hypertensive injury. This may be the case particularly in the early post-revascularization period, when the hypotensive effect of angioplasty may not yet be apparent and the “protective” effect of the renal stenosis against hypertensive kidney damage is no longer present. This theory could, at least in part, explain the unsatisfactory improvement of kidney filtration function following RAS revascularization. Regarding the impact of revascularization on kidney function, previous reports have shown that improvements in renal function are observed only in some individuals undergoing renal revascularization and that deterioration of renal function after intervention occurs in approximately 30% of patients (Citation20,Citation21). We assume that qualification for RAS revascularization may require not only evaluation of the hemodynamic significance of the stenosis by induction of hyperemia but also assessment of the ability of afferent arterioles for proper tone adjustment, especially during the post-procedural period.
Study limitations
We realize the important limitations of this non- randomized study, which was based on the observation of only 12 cases. We also realize that further investigations, especially assessment of the influence of afferent arteriole reactivity on long-term renal filtration function, may be difficult and time consuming as recruitment of patients with RAS is challenging. However, we believe that future studies on renal revascularization should include the assessment of kidney microcirculation function.
Conclusions
The study shows an easy method for assessment of the response of the renal microcirculation to vasoconstriction by pharmacological agents. We showed that in hypertensive patients with RAS, the vasomotor activity of afferent arterioles may be preserved. The role of afferent arteriole reactivity in predicting a favorable outcome from renal revascularization, especially its influence on long-term renal filtration function, was not the purpose of the study and, therefore, was not analyzed. The significance of the influence of adenosine on translesional gradients should be determined in future studies.
Funding
This study was partially supported by Medical University of Wroclaw scientific grant no. 1639.
Declaration of interest: We have no shares or interests in companies marketing, or who have marketed therapies that might be covered in this article.
References
- Subramanian R, White CJ, Rosenfield K, Bashir R, Almagor Y, Meerkin D, Shalman E. Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenosis. Catheter Cardiovasc Interv. 2005;64:480–6.
- Siddiqui TS, Elghoul Z, Reza ST, Leesar MA. Renal hemodynamics: theory and practical tips. Catheter Cardiovasc Interv. 2007;69:894–901.
- Mitchell JA, Subramanian R, White CJ, Soukas PA, Almagor Y, Stewart RE, Rosenfield K. Predicting blood pressure improvement in hypertensive patients after renal artery stent placement. Catheter Cardiovasc Interv. 2007;69:685–9.
- Mangiacapra F, Trana C, Sarno G, Davidavicius G, Protasiewicz M, Muller O, et al. Translesional pressure gradients to predict blood pressure response after renal artery stenting in patients with renovascular hypertension. Circ Cardiovasc Interv. 2010;3:537–42.
- Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol. 2003;285:590–9.
- Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L’Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res. 1996;32:949–53.
- Wierema TK, Postma CT, Houben AJ, Kroon AA, Thien T, Smits P, de Leeuw PW. Adenosine-induced renal vasodilatation is prolonged in renal artery stenosis. J Hypertens. 1998;16:2109–12.
- Spielman WS, Thompson CI. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol Renal Physiol. 1982;242(5):F423–35.
- Osswald H, Schmitz HJ, Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflugers Arch. 1975;357:323–33.
- Tagawa H, Vander AJ. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ Res. 1970; 26:327–38.
- Thurau K. Renal hemodynamics. Am J Med. 1964;36: 850–60.
- Joyner WL, Mohama RE, Myers TO, Gilmore JP. The selective response to adenosine of renal microvessels from hamster explants. Microvasc Res. 1988;35:122–31.
- Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Vasomotor effects of purinergic agonists in isolated rabbit afferent arterioles. Am J Physiol Renal Fluid Electrolyte Physiol. 1992;263:1026–33.
- Leesar MA, Varma J, Shapira A, Fahsah I, Raza ST, Elghoul Z, et al. Prediction of hypertension improvement after stenting of renal artery stenosis: comparative accuracy of translesional pressure gradients, intravascular ultrasound, and angiography. J Am Coll Cardiol. 2009;53:2363–71.
- Protasiewicz M, Kądziela J, Początek K, Poręba R, Podgórski M, Derkacz A, et al. Renal artery stenosis in patients with resistant hypertension. Am J Cardiol. 2013;112:1417–20.
- Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand. 2004;181:407–13.
- Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res. 2002; 90:1316–1324.
- Hartman ME. The relationship of glomerular plasma flow to the etiology and progression of hypertension. Anat Rec. 1960;138:67–72.
- Goldblatt H, Lynch J, Hanzal RJ, Summerville WW. Studies on experimental hypertension I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–9.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int. 1998;53:799–811.
- Harden PN, MacLeod MJ, Rodger RSC, Baxter GM, Connell JMC, Dominiczak AF, et al. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–6.