Abstract
The microvasculature and macrovasculature undergo extensive, organ-specific perinatal maturation. Multiple studies show associations between low birth weight and subsequent cardiovascular dysfunction in adulthood, suggesting that extreme preterm birth interferes with this maturation process. Therefore, we designed PREMATCH (PREMATurity as predictor of Cardiovascular–renal Health) to phenotype the microcirculation and macrocirculation during childhood in former preterm infants. A well-characterized cohort of former extreme preterm birth survivors and gender- and age-matched controls (aged 8–13 years) will be investigated for microvascular and macrovascular structure and function. In addition to cognitive performance and anthropometrics, we will investigate (i) the microvascular structure and function by endothelial function (photoplethysmography), sublingual capillary glycocalyx function (sidestream dark field imaging) and retinal structure (diameters of arterioles and venules); and (ii) the macrovascular phenotype by cardiac and renal ultrasound, repeated blood pressure measurements and arterial pulse-wave recordings. The PREMATCH study is unique in its design, and ongoing recruitment demonstrates excellent feasibility. The expectation is that the results of this study will identify risk factors during childhood for subsequent cardiovascular–renal disease in the adult life of former preterm infants, while further analysis on mediators in neonatal life of this cardiovascular–renal outcome may provide new information on perinatal risk factors.
Trial registration: ClinicalTrials.gov identifier: NCT02147457.
Introduction
The cardiovascular system undergoes extensive maturation throughout fetal, perinatal and pediatric life. Extreme preterm birth (i.e. extremely low birth weight, ELBW, birth weight < 1000 g) interferes with normal development of the cardiovascular system and other organs, resulting in suboptimal development (Citation1–4). There is evidence that extreme preterm birth increases the cardiovascular risk profile in adulthood (Citation5). In essence, this may be a reflection of the Barker hypothesis, or fetal origin hypothesis (Citation1), which states that a preterm infant, similarly to a growth-restricted newborn, does not reach its genetic potential for growth, leading to affected development of the microcirculation and macrocirculation.
Preterm birth interrupts the fetal conditions too early, potentially leading to maldevelopment of the microvascular structure and circulation in various organs, such as the retina [resulting in retinopathy of prematurity (Citation6)] and the kidney [abnormal glomerulogenesis (Citation7)]. Epidemiological observations further confirm that infants born preterm display a higher risk than full-term infants of developing cardiovascular disease and chronic kidney disease during adulthood (Citation1,Citation2,Citation8–10).
Rationale and design
Improved care for extreme preterm infants in neonatal intensive care units has led to the increased survival of these infants and an increased population of former preterm adults. Cardiovascular abnormalities following preterm birth include hypertension (Citation10), but evidence concerning endothelial dysfunction is not consistent (Citation11,Citation12).
The PREMATurity as predictor of Cardiovascular–renal Health (PREMATCH) study aims to move beyond the state of the art by simultaneously assessing both the microvascular and macrovascular structure and function in children (8–13 years old) in a case–control design. The index children are former ELBW infants, who each will be matched for gender and age with two controls. A particular strength of this study is that this ELBW cohort has been well characterized with respect to its perinatal aspects (Citation13) (e.g. birth characteristics, growth pattern, perinatal drugs, respiratory support, Apgar scores, retinopathy). We will investigate the association of these perinatal aspects with current cardiovascular–renal health when the former preterm infants are currently 8–13 years old. Furthermore, a broader phenotyping regarding general health (level of current education, grip strength, visual acuity) will be performed. In addition, we will examine routine clinical variables (i.e. anthropometrics, questionnaire). Finally, a cognitive performance test will complete the phenotyping.
Hypothesis and objectives
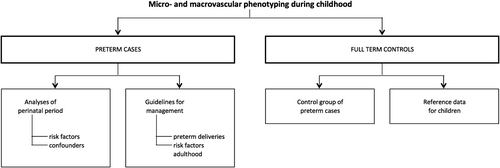
We hypothesize that former ELBW infants, compared to controls following term birth, will have marked alterations in their microcirculatory and macrocirculatory structure and function, even at this young age. These changes, even if subtle, are probably forerunners of cardiovascular–renal disease in adulthood. In addition, confounders (e.g. neonatal nutrition, neonatal treatment) documented in early life may identify new approaches for timely prevention ().
Materials and methods
Participants
Our cohort consists of 140 extreme preterm birth survivors (ELBW, birth weight < 1000 g) (Citation13) who each will be matched for gender and age with two controls. Cases have the opportunity to bring an age- and gender-matched friend with them, who will be the first control subject. A second control will be recruited in the neighborhood of the research center (Eksel, Belgium).
Ethics
The study will be conducted in accordance with the Helsinki Declaration for Investigation in Human Subjects. The local ethics committee of the University Hospitals Leuven (Belgium) approved the study protocol (June 2014, S56577). Based on good clinical practice guidance and national law, parents or custodians will provide written informed consent, while the child will have to give informed assent. The study is registered at ClinicalTrials.gov (NCT02147457).
Protocol
We will investigate the microvascular and macrovascular structure and function and additional phenotyping (i.e. cognitive performance, anthropometrics, etc.) in children born preterm and healthy controls (defined as term gestation). schematically represents how PREMATCH intends to move from risk factors and measurement of phenotypes to recommendations for clinical care.
Microcirculatory phenotyping
The microcirculation will be assessed functionally (endothelial function, glycocalyx measurements) and structurally (retinal imaging).
Endothelial function will be assessed by 24 h urinary microalbumin excretion and by the digital pulse-wave amplitude hyperemic response, measured by photoplethysmography (PPG; Flomedi, Brussels, Belgium). The absolute intraobserver variability in amplitude ratios in our hands is 0.062 for the average PPG and 0.072 for the PPG pulse; the interobserver variability is 3.29% in the average PPG pulse and 4.87% in the peak PPG pulse (Citation14).
Capillary glycocalyx and density will be measured in sublingual capillaries in two areas lateral to the frenulum and 3–4 cm anterior to the tongue base. To visualize these capillaries, we will use the Handheld Video Capillary Microscope (KK Research Technology, Honiton, UK), interfaced with a laptop computer running the GlycoCheck® software (GlycoCheck, Maastricht, The Netherlands) (Citation15). After adequate recording by sidestream dark-field (SDF) imaging, the software calculates the perfused boundary region (PBR), which reflects the thickness of the endothelial glycocalyx, based on the assumption that loss of its integrity allows deeper penetration of red blood cells into the gel-like layer covering the endothelial lining. Higher PBR therefore indicates thinner glycocalyx. The software also returns total and perfused capillary density in segments per square millimeter (Citation15).
Retinal imaging will be performed using a Canon Cr-DGi (Canon Co., Kyoto, Japan) non-mydriatic retinal visualization system. After accommodation to darkness, one image per eye will be obtained (Citation16). Trained observers will identify individual arterioles and venules using a validated computer-assisted program, IVAN (Vasculo-matic Nicola, Ophthalmology and Visual Science, University of Wisconsin-Madison, USA) (Citation17). This software combines the individual measurements into summary indices: central retinal arteriolar equivalent (CRAE), central retinal venular equivalent (CRVE) and arteriole-to-venule ratio (AVR), based on published validated formulae (Citation18–20). Our intraobserver and interobserver variability are 13.2% and 10.8% for CRAE, 8.4% and 9.9% for CRVE, and 9.0% and 14.6% for AVR, respectively (Citation16). In addition, we will investigate visual acuity (clearness of vision, spatial resolution of the visual processing system) using the non-invasive adapted Snellen charts (Medical Workshop, Groningen, The Netherlands), with and without visual aids.
Macrocirculatory phenotyping
Similarly to the microcirculation, the macrocirculation will be assessed structurally (echocardiography, renal ultrasound and ultrasound of the carotid artery) and functionally (central hemodynamics, blood pressure).
Two experienced sonographers will perform a detailed echocardiographic examination in all children, using a Vivid E9 device (GE Vingmed, Horten, Norway), interfaced with a 2.5–3.5 MHz phased-array probe. The digitally stored images will be analyzed with EchoPac software (GE Vingmed). The acquisitive and offline analysis of the M-mode, two-dimensional tissue Doppler and left ventricular images will be carried out according to the recommendations of the American Society of Echocardiography (Citation21). Because of maturational effects and growth characteristics, Z- scores will be calculated according to published reference values (Citation22).
Renal anatomy will be assessed by two-dimensional ultrasound imaging (Vivid E9 interfaced with a 2.4–10 MHz linear array probe) and renal arterial Doppler blood flow measurements. Again, ultrasound measurements are dependent on age and more importantly length, so Z-scores will be used to compare our children to their controls and to the general child population according to published reference values (Citation23).
Based on imaging of the common carotid arteries by ultrasound, we will derive the carotid artery diameter, distension and intima–media thickness. Subsequently, carotid distensibility (103/kPa), compliance (mm²/kPa) and the Young's elastic modulus will be calculated.
Central hemodynamics will be calculated from peripheral arterial pulse-wave recordings. Pulse waves will be recorded at the carotid, radial and femoral arteries, by local application tonometry (SPC-301 micromanometer; Millar Instruments, Houston, TX, USA). From these recordings, the aortic pulse wave can be reconstructed by means of a validated generalized transfer function (SphygmoCor®, version 7.1; AtCor, Sydney, Australia) and the central blood pressure and the systolic augmentation index can be calculated. Finally, the pulse-wave recording at three different sites will allow us to calculate the carotid-to-femoral and carotid-to-radial pulse-wave velocities.
During a home visit, the blood pressure will be measured by the auscultatory Korotkoff approach using a mercury-free device (UM-101A; A&D Medical San Jose, USA) after the participants have rested for 5 min in the sitting position.
Additional phenotyping
Renal function (glomerular filtration rate) will be retrieved from the measurement of creatinine clearance and 24 h microalbuminuria (24 h urine collection) and cystatin C (particle-enhanced turbidimetric immunoassay, Integra 400 system; Roche Diagnostics, Basel, Switzerland) in peripheral blood (serum).
Body composition will be examined by trained staff using the Bodystat QuadScan 4000 (Bodystat, Isle of Man, British Isles). This device applies scientifically validated principles of bioelectrical impedance (Citation24,Citation25) and provides detailed information on a large number of variables, including lean and fat-free mass, body-fat mass index, fat-free mass index and the distribution of total body water over the intracellular and extracellular space.
Cognitive performance will be assessed using the Wechsler Non-Verbal test, Dutch version (Pearson, Amsterdam, The Netherlands). We will examine both matrix reasoning and spatial span.
Body weight, muscle mass, fat mass, body mass index (BMI) and resting metabolism will be examined using the Omron Karada Scan (HBF511; Omron, Kyoto, Japan).
Puberty scores will be assessed using the Tanner scale (Citation26,Citation27).
Waist, hip, head and neck circumference will be measured using a tape measure. The umbilicus will be used as a marker of waist circumference. The greater trochanter will be used as a landmark for the hip circumference. Head circumference will be measured with a tape measure on the forehead and around the full circumference of the head. Neck circumference will be measured just below the larynx (thyroid cartilage) and perpendicular to the long axis of the neck. The participant will be asked to look straight ahead. Care will be taken not to involve the shoulder/neck muscles (trapezius) in the measurement of the neck circumference. Height and span width will also be examined. For the span width, the participant stands with the back against a wall, spreading the arms while placing the fingertips of one hand against a bar. The length will be measured with a ruler.
The Jamar Hydraulic Hand Dynamometer (Sammons Preston, Chicago, IL, USA) will be used to measure handgrip strength according to the owner's manual. After setting the adjustable handle to the desired spacing, the red peak-hold needle will be set to zero. In a sitting position with the elbow flexed at 90° and the forearm and wrist in a neutral position, the participant will be asked to squeeze with maximum strength. This procedure will be repeated three times.
A caliper will be used to measure triceps, subscapular and suprailiac skinfolds (Harpenden Skinfold Caliper; John Bull, Bedfordshire, UK). This caliper will provide a constant pressure. Measurements will be read after 3 s to two decimal places.
Parents will be asked to complete a questionnaire regarding the health, educational level and medication use of their child. In addition, parents will be asked for their familial medical history, their educational level and BMI, and environmental factors concerning the child (e.g. smoking in the vicinity of the child).
Children will be asked to collect 24 h urine samples (volume, electrolytes, creatinine, microalbumin, aldosterone) and to provide a blood sample. Routine analysis and storage for markers of metabolic stress (e.g. interleukin-6, high-sensitivity C-reactive protein) will be performed. Blood sampling will be performed after fixation of a Rapydan® patch (70 mg lidocaine/ 70 mg tetracaine; Eurocept, Ankeveen, The Netherlands) to minimize discomfort. In addition, we will ask parents for additional consent concerning hereditary studies (blood sample of parents for DNA to study known cardiovascular risk genes) to see whether the differences between cases and controls are due to their gestational age (ELBW, control) or could have a genetic origin.
GPS coordinates of residence will be measured, and meteorological data and data on airborne pollutants and fine particulates will be collected from the appropriate sources.
Database construction and statistical analysis
For database management and statistical analysis, we will use the SAS and SPSS system (two-tailed significance level < 0.05). Means, proportions and Kaplan–Meier survival function estimates will be compared using the large-sample Z test, analysis of variance (ANOVA), Fisher's exact test or the log-rank test. If required, the distribution of variables will be normalized by appropriate transformation. Methods will also include multivariable adjusted linear and logistic regression analysis for cross-sectional data, and linear regression and proportional hazards regression for longitudinal data. Stepwise regression procedures (p value for entry and removal set at 0.15) will be applied to identify potential important covariables. We will search for optimized discrimination limits for predictive baseline factors by maximizing Youden's index. In addition, we will assess improvement in predictor variables, beyond what is currently known, by computing the integrated discrimination improvement and the net reclassification improvement (Citation28,Citation29).
Data from questionnaires will be collected online, preferably through computer-assisted self-interviewing (CASI) or computer-assisted telephone interviewing (CATI) before scheduled home visits, or computer-assisted personal interviewing (CAPI) during home visits, using MOTUS software (designed by the Research Group TOR, Sociology Department, Vrije Universiteit Brussel, Belgium, www.prematch.be).
Results
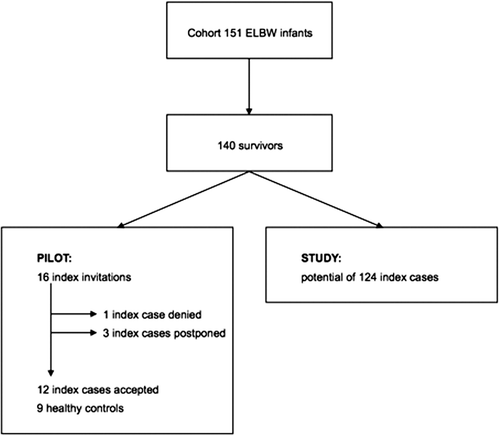
To prove feasibility, a pilot study started in October 2014 and was completed in December 2014. Sixteen former preterm infants were invited. Only one child declined participation, and three preferred to participate in a later phase of the study. Three children were not yet matched to a control. In total, 12 cases and nine controls were fully investigated. The current participation rate of the former preterm infants was 75%. shows a flowchart of PREMATCH.
The characteristics of the patients are summarized in . Basic results of microcirculation and macrocirculation and general investigations are summarized in . Statistical analysis was not performed owing to the low numbers and the goal of this article (showing feasibility and study design). These results show that the phenotyping was tolerated and data collection was feasible in all examined patients. A survey of the pilot children revealed no major discomforts reported in examining the children (). The response rate of the questionnaire was 63%. In addition, parents reported high acceptability of the efforts requested (24 h urine collection, traveling to the research center) ().
Table I. Characteristics of participants in the pilot study.
Table II. Phenotyping of participants in the pilot study.
Table III. Survey of discomfort and participation efforts in the pilot study.
Discussion
A cohort of 140 former preterm infants will be characterized at the age of 8–13 years in a case–control design and the data will be related to those collected during the postnatal period. This additional retrospective cohort analysis adds a risk analysis in former preterm infants in reference to the control population. We will investigate perinatal risk factors such as the use of ibuprofen, ventilation days and intraventricular hemorrhage. In addition, we have collected information about the pregnancy [pre-eclampsia, tocolysis, lung maturation in utero, premature rupture of membranes (Citation13)] and these data can add further value to the risk analysis. If applicable, we will include data on the placental pathology. The phenotypes of interest in the case–control study include the microcirculatory and macrocirculatory and cardiac structure and function, endothelial function and renal anatomy and function during childhood. The expectation is that the results of this study will identify and quantify risk factors for cardiovascular–renal disease in former preterm infants compared to control children, while we can also map early risk factors for cardiovascular–renal disease in adult life and pave the way for better informed prevention of these complications. As preterm infants are more often surviving the postnatal period, the long-term outcome and secondary prevention become of extreme importance. The current cohorts of former preterm infants do not undergo investigation of both microvasculature and macrovasculature, and adult studies show that this is of utmost importance (Citation30,Citation31). The PREMATCH study is unique in its design, and ongoing recruitment demonstrates excellent feasibility.
If we compare our cohort of former preterm infants to cohorts reported in the literature, there are some important methodological differences related to inclusion criteria (birth weight) and assessments made (both microvasculature and microvasculature). We did not discriminate upon gestational age of the former preterm infant, but we did investigate children with ELBW (< 1000 g), whereas, for example, the EPICure cohort (Citation32) studies included infants in between 22 and 26 weeks of gestational age, independent of birth weight. As a consequence, PREMATCH will have a larger number of children small for gestational age, owing to the study design, but will therefore have an excellent study design to investigate the prenatal and postnatal growth restriction seen in premature infants, reflecting the Barker hypothesis (i.e. growth restriction). The EPIPAGE cohort (Etude Epidémiologique sur les Petits Ages Gestationnels) (Citation33) and POPS cohort (Project On Preterm and Small-for-gestational-age infants) (Citation34) consist of a broader mixture of gestational ages (22–32 weeks gestational age).
Besides differences in inclusion criteria, we aim to address aspects of the microvasculature and microvasculature that have not yet been systematically documented in such a specific cohort of ELBW infants. The EPICure cohort in the UK mainly focuses on neurocognitive outcome and lung function (Citation35–37), while the EPIPAGE cohort shows comprehensive data collection on neurocognitive outcome and infectious characteristics (Citation38,Citation39). Whereas the first cohorts of preterm infants are now adults [POPS cohort, The Netherlands (Citation34)] and those studies mainly focus on neurocognitive outcome and endocrinological disturbances (Citation40,Citation41), we are trying to find early markers of cardiovascular dysfunction and to focus on long-term health in an era after major improvements in neonatal intensive care. Finally, the POPS study also provided evidence of microalbuminuria in young adulthood (mean age at evaluation 19 years) after former preterm birth (Citation42).
Different aspects of long-term outcome and the consequences of prematurity should be evaluated. Neurocognitive outcome remains the most studied object in former preterm infants (Citation37,Citation38,Citation41,Citation43–47). While other cohorts also focus on lung function (Citation36), renal function (Citation9) or cardiovascular risk, e.g. blood pressure (Citation4,Citation48) or intima media thickness (Citation49), we will try to investigate simultaneously microvascular and macrovascular changes and explore the interactions between risk factors and early biomarkers. A specific strength of this study is that the preterm cohort and their mothers have already been well characterized in the perinatal period (Citation13).
Such long-term outcome studies are hampered by the challenges relating to technical investigations in children, including feasibility and validity. For example, endothelial function measured in children can be difficult and results can be confusing. Measuring endothelial function before and after transdermal acetylcholine revealed no significant differences in children born preterm in comparison to a healthy control population (Citation11). This may be due to the lack of power (n = 39), because the results seem not to be consistent (Citation50). The most common uncertainties in measuring endothelial function in a non-invasive way are the local, environmental and other factors that can modulate the measured shear stress. For example, younger age can influence reactive hyperemia (Citation51). The same group showed that flow-mediated dilatation is less vulnerable to such influences (Citation51). In addition, the growth pattern after birth can have an influence on the endothelial function (Citation52), but since we have a well-characterized cohort, we can correct for such influences as well. Another example is the measurement of the glycocalyx by SDF or orthogonal polarization spectral (OPS) imaging. Both techniques have comparable properties; SDF is the modified version of the OPS (Citation53). It has been shown that SDF is a feasible microperfusion marker in healthy adults (Citation15). In children with type 1 diabetes mellitus, these microvascular alterations can be detected early and more specifically, before apparent vascular complications (Citation54). Although this may be an acquired alteration in these children instead of a structural problem in former preterm infants, these results at least show the feasibility of detecting alterations before clinical complications. In addition, former preterm infants have an increased risk of cardiovascular complications and, as the Barker hypothesis suggests (Citation1), this risk may be attenuated by the presence of impaired glucose tolerance. The last example is retinal imaging, were there is already increasing evidence that these images can predict cardiovascular risk in specific subpopulations of children (Citation55–58). It has been shown that there is an association with BMI and weight (Citation56), type 1 diabetes in children (Citation55) and blood pressure (Citation57,Citation59). In addition, there is evidence that low birth weight is associated with significant changes in the microvasculature of the retina (Citation58,Citation60). The strength of PREMATCH is that microvascular data are combined with macrovascular data and additional phenotyping (i.e. anthropometrics, cognitive performance) to obtain a grip on risk factors and confounders of ELBW. In addition, we showed excellent feasibility, with no discomfort reported or objectified ().
To analyze our data, we will use Z-scores whenever possible. A Z-score is a standard deviation score of a measured value (e.g. kidney size) as a distance between the child's value and the expected value (we will use published reference data for children if possible), with a mean of zero and a standard deviation of 1. Since reference data are not routinely available for the experimental measurements of microvascular and macrovascular function, we will generate a larger group of control children to create reference data to calculate Z-scores for further studies. We are using Z-scores because of their major advantages, compared to percentiles for example, in that these Z- scores reflect the reference distribution and are standardized and thus comparable across age, gender and anthropometric measurements. For former preterm infants, we can also quantify the distribution at the end of the spectrum. Another advantage is that we can analyze Z-scores as a continuous variable.
Although our study may be considered extensive and invasive, and consequently ambitious, we have shown in a pilot study an excellent feasibility and participation rate (at least 75%). In case of a lower participation rate in the sequel to the study, or midterm analysis revealing larger sample size calculations, we will consider pooling our cohort with neonatal intensive care units with which we are in collaboration (Citation61).
Since our cohort has been characterized in detail in the postnatal period (Citation13), potential confounders of preterm birth can be subcategorized and analyzed, and we may be able not only to detect differences between cases and controls, but also to point out risk factors in the perinatal period. Our ultimate goal is to improve guidelines for neonatal care.
Finally, pooling of cardiovascular phenotypic data in children with other cohorts may provide opportunities for additional prediction model development, validation and subsequent secondary preventive strategies ().
Acknowledgments
The authors gratefully acknowledge the expert assistant of the field center (Eksel, Belgium) technicians (Linda Custers, Marie-Jeanne Jehoul, Daisy Thijs and Hanne Truyens) and the administrative assistance of Annick De Soete (Studies Coordinating Centre, Leuven, Belgium).
Funding
The European Union [HEALTH-2011.2.4.2-2-EU-MASCARA, HEALTH-F7-305507 HOMAGE and the European Research Council Advanced Researcher Grant-2011-294713-EXPLORE] and the Fund for Scientific Research Flanders [G.0881.13 and G.088013] currently support the Studies Coordinating Centre in Leuven. This study was supported by the Agency for Innovation by Science and Technology in Flanders (IWT) through the SAFE-PEDRUG project [IWT/SBO 130033]. KA and EL are senior clinical investigators of the Fund for Scientific Research, Flanders [Fundamental Clinical Investigatorship, 1800214N and 1801110N] and EL is also supported by the EURenOmics consortium [grant agreement 305608].
Declaration of interest: None of the authors declares a conflict of interest.
References
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4.
- Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–79.
- Keijzer-Veen MG, Devos AS, Meradji M, Dekker FW, Nauta J, van der Heijden BJ. Reduced renal length and volume 20 years after very preterm birth. Pediatr Nephrol. 2010;25: 499–507.
- Kerkhof GF, Breukhoven PE, Leunissen RW, Willemsen RH, Hokken-Koelega AC. Does preterm birth influence cardiovascular risk in early adulthood? J Pediatr. 2012;161:390–6.e1.
- Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol. 2011; 40:647–61.
- O’Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–8.
- Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–74.
- Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8:265–74.
- Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci. 2011;18:322–33.
- Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013; 131(4):e1240–63.
- Bonamy AK, Martin H, Jorneskog G, Norman M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med. 2007;262:635–42.
- Norman M, Martin H. Preterm birth attenuates association between low birth weight and endothelial dysfunction. Circulation. 2003;108:996–1001.
- George I, Mekahli D, Rayyan M, Levtchenko E, Allegaert K. Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol. 2011; 26:1843–9.
- Kuznetsova T, Szczesny G, Thijs L, Jozeau D, D'hooge J, Staessen JA. Assessment of peripheral vascular function with photoplethysmographic pulse amplitude. Artery Res. 2011; 5:58–64.
- Hubble SM, Kyte HL, Gooding K, Shore AC. Variability in sublingual microvessel density and flow measurements in healthy volunteers. Microcirculation. 2009;16:183–91.
- Liu Y-P, Richart T, Jin Y, Struijker-Boudierc HA, Staessen JA. Retinal arteriolar and venular phenotypes in a Flemish population: reproducibility and correlates. Artery Res. 2011;5: 72–9.
- Sherry LM, Wang JJ, Rochtchina E, Wong T, Klein R, Hubbard L, et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin Exp Ophthalmol. 2002; 30:179–82.
- Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–80.
- Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77:472–7.
- Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol. 1974;77:478–83.
- Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119.
- Dallaire F, Slorach C, Hui W, Sarkola T, Friedberg MK, Bradley TJ, et al. Reference values for pulse wave Doppler and tissue Doppler imaging in pediatric echocardiography. Circ Cardiovasc Imaging. 2015;8(2):e002167.
- Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611–6.
- Segal KR, Burastero S, Chun A, Coronel P, Pierson RN Jr, Wang J. Estimation of extracellular and total body water by multiple-frequency bioelectrical-impedance measurement. Am J Clin Nutr. 1991;54:26–9.
- Stewart SP, Bramley PN, Heighton R, Green JH, Horsman A, Losowsky MS, et al. Estimation of body composition from bioelectrical impedance of body segments: comparison with dual-energy X-ray absorptiometry. Br J Nutr. 1993;69:645–55.
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303.
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23.
- Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802.
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72; discussion 207–12.
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–6.
- Kumagai K, Tabara Y, Yamashiro K, Miyake M, Akagi-Kurashige Y, Oishi M, et al. Central blood pressure relates more strongly to retinal arteriolar narrowing than brachial blood pressure: the Nagahama Study. J Hypertens. 2015; 33:323–9.
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–84.
- Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr. 2003;143:477–83.
- Veen S, Ens-Dokkum MH, Schreuder AM, Verloove-Vanhorick SP, Brand R, Ruys JH. Impairments, disabilities, and handicaps of very preterm and very-low-birthweight infants at five years of age. The Collaborative Project on Preterm and Small for Gestational Age Infants (POPS) in The Netherlands. Lancet. 1991;338:33–6.
- Bolton CE, Stocks J, Hennessy E, Cockcroft JR, Fawke J, Lum S, et al. The EPICure study: association between hemodynamics and lung function at 11 years after extremely preterm birth. J Pediatr. 2012;161:595–601.e2.
- Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–45.
- Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and2006: the EPICure studies.BMJ 2012;345:e7961.
- Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, et al. Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011;100:370–8.
- Mitha A, Foix-L’Helias L, Arnaud C, Marret S, Vieux R, Aujard Y, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013; 132:e372–80.
- Meuwese CL, Euser AM, Ballieux BE, van Vliet HA, Finken MJ, Walther FJ, et al. Growth-restricted preterm newborns are predisposed to functional adrenal hyperandrogenism in adult life. Eur J Endocrinol. 2010;163:681–9.
- Teune MJ, van Wassenaer AG, van Dommelen P, Mol BW, Opmeer BC. Perinatal risk indicators for long-term neurological morbidity among preterm neonates. Am J Obstet Gynecol. 2011;204:396.e1–14.
- Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol. 2005;16:2762–8.
- Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92.
- Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonatal Med. 2014;19:97–104.
- Larroque B, Ancel PY, Marchand-Martin L, Cambonie G, Fresson J, Pierrat V, et al. Special care and school difficulties in 8-year-old very preterm children: the Epipage cohort study. PloS One. 2011;6(7):e21361.
- Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19.
- Weisglas-Kuperus N, Hille ET, Duivenvoorden HJ, Finken MJ, Wit JM, van Buuren S, et al. Intelligence of very preterm or very low birthweight infants in young adulthood. Arch Dis Child Fetal Neonatal Ed. 2009;94:F196–200.
- Norberg H, Stalnacke J, Nordenstrom A, Norman M. Repeat antenatal steroid exposure and later blood pressure, arterial stiffness, and metabolic profile. J Pediatr. 2013;163:711–6.
- Schubert U, Muller M, Abdul-Khaliq H, Norman M, Bonamy AK. Relative intima–media thickening after preterm birth. Acta Paediatr. 2013;102:965–9.
- Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102:2739–44.
- Kelly AS, Marlatt KL, Steinberger J, Dengel DR. Younger age is associated with lower reactive hyperemic index but not lower flow-mediated dilation among children and adolescents. Atherosclerosis. 2014;234:410–4.
- Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109:1108–13.
- Awan ZA, Wester T, Kvernebo K. Human microvascular imaging: a review of skin and tongue videomicroscopy techniques and analysing variables. Clin Physiol Funct Imaging. 2010;30:79–88.
- Nussbaum C, Cavalcanti Fernandes Heringa A, Mormanova Z, Puchwein-Schwepcke AF, Bechtold-Dalla Pozza S, Genzel-Boroviczeny O. Early microvascular changes with loss of the glycocalyx in children with type 1 diabetes. J Pediatr. 2014;164:584–9.e1.
- Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31: 1842–6.
- Cheung N, Saw SM, Islam FM, Rogers SL, Shankar A, de Haseth K, et al. BMI and retinal vascular caliber in children. Obesity. 2007;15:209–15.
- Gopinath B, Baur LA, Wang JJ, Teber E, Liew G, Cheung N, et al. Blood pressure is associated with retinal vessel signs in preadolescent children. J Hypertens. 2010;28:1406–12.
- Gopinath B, Baur LA, Wang JJ, Teber E, Liew G, Cheung N, et al. Smaller birth size is associated with narrower retinal arterioles in early adolescence. Microcirculation. 2010;17: 660–8.
- Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FM, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156–62.
- Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N, et al. Evidence of arteriolar narrowing in low-birth-weight children. Circulation. 2008;118:518–24.
- Van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwe W, Jespers A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1945–9.