Abstract
Abnormal night-time blood pressure (BP) reduction is associated with increased cardiovascular risk in hypertension. Little is known about the prevalence and covariates of night-time BP reduction in ischemic stroke patients. Clinic and ambulatory BP measurements were recorded in 268 stroke survivors aged 15–60 years. The degree of night-time dipping was calculated from the difference between day-time and night-time mean BP, and defined as non-dipping if < 10%. Aortic stiffness was derived from carotid–femoral pulse-wave velocity (PWV) by applanation tonometry and carotid intima–media thickness (cIMT) by ultrasound. A non-dipping pattern was found in 38%. Non-dippers had higher PWV, mean cIMT and night-time BP, and included more patients with history of hypertension, diabetes and high for age PWV compared to dippers (all p < 0.05). In multivariate logistic regression analyses, non-dipping was associated with high for age PWV [odds ratio (OR) = 2.28; 95% confidence interval (CI) 1.06–4.92, p < 0.05] independent of history of hypertension and other confounders, while elevated night-time BP was associated with increased cIMT (OR = 3.83; 95% CI 1.01–14.50, p < 0.05) independent of non-dipping status, male gender, obesity, antihypertensive treatment and high for age PWV. In conclusion, in the Norwegian Stroke in the Young Study, non-dipping BP pattern was common and associated with increased aortic stiffness.
Introduction
In healthy individuals, blood pressure (BP) declines during night-time sleep by approximately 10–20%. A night-time BP reduction less than 10% is referred to as non-dipping. The cause of abnormal night-time BP reduction is often multifactorial,[Citation1,Citation2] and may be related to sleep disorders, altered autonomic nervous system function, disturbed baroreflex sensitivity, environmental stressors and comorbidities, or the interaction of these factors in the individual patient.[Citation3,Citation4] Several previous publications have pointed out the association of abnormal night-time BP reduction with increased risk for cardiovascular events in general as well as in hypertensive populations.[Citation5–8] In older stroke patients, lack of night-time BP reduction has been associated with lower cerebral circulation [Citation9] and worse prognosis.[Citation10] Taken together, the evidence suggests that elevated night-time BP may be a marker of atherosclerosis.[Citation11]
In clinical practice, atherosclerosis may be suspected from elevated aortic pulse-wave velocity (PWV) or increased carotid intima–media thickness (cIMT), reflecting functional and structural arterial changes.[Citation11] Increased PWV was recently reported to be common also among younger ischemic stroke patients,[Citation12] and particularly associated with clustering of cardiovascular risk factors.[Citation12] The aim of the present study was to explore the associations of non-dipping BP pattern and night-time BP with arterial stiffening and remodeling measured by PWV and cIMT in ischemic stroke patients aged ≤ 60 years participating in the Norwegian Stroke in the Young Study (NOR-SYS).
Methods
Study population and design
The prospective NOR-SYS research program is conducted at the Department of Neurology, Haukeland University Hospital, Bergen, Norway, and includes patients aged 15–60 years with acute ischemic stroke verified by cerebral magnetic resonance imaging. The rationale and study design of NOR-SYS have been previously published.[Citation13] All patients were scheduled for cardiovascular work-up including 24 h ambulatory BP recording and assessment of arterial stiffness. Between September 2010 and January 2015, a total of 344 patients was recruited. For the present analysis, patients who underwent technical successful recording of ambulatory BP and aortic PWV were included. Eighteen patients were excluded owing to the presence or history of atrial fibrillation, 34 patients owing to missing or technical inadequate ambulatory BP recording and 24 owing to missing or technically inadequate PWV analyses. Death and serious sequelae of ischemic stroke were the main reasons for dropout.
The study was approved by the Regional Committee for Medical Research Ethics of Western Norway, and conducted in accordance with the Declaration of Helsinki. All patients or their legal representatives signed written informed consent.
Metabolic risk factors
A standardized questionnaire was used to record self-reported information about cardiovascular risk factors and drug use. Body mass index (BMI) was calculated from body weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥ 30 kg/m2. Venous blood samples were drawn for analysis of serum lipids, glucose, sodium, potassium and creatinine. Hypercholesterolemia was considered present if total serum cholesterol was > 5.0 mmol/l and/or low-density lipoprotein (LDL) cholesterol > 3.0 mmol/l.[Citation14] Low high-density lipoprotein (HDL) cholesterol was defined as < 1.0 mmol/l (men) and 1.3 mmol/l (women).[Citation14]
The presence of the metabolic syndrome was defined using the modified American Heart Association/National Heart, Lung, and Blood Institute criteria.[Citation15] Diabetes mellitus was defined as a history of diabetes, use of antidiabetic treatment or fasting blood glucose ≥ 7.0 mmol/l.[Citation16].
Office and ambulatory blood pressure
Clinic BP measurements were performed 1 month after the index stroke on the same day as the measurement of arterial stiffness. Brachial BP was measured in triplicate by the same investigator using regularly calibrated aneroid sphygmomanometers and appropriate cuff size after 5 min rest in the sitting position.[Citation17] The average of the last two measurements was taken as the brachial BP. Hypertension was defined as a reported history of hypertension at the admission for the index stroke, or elevated clinic and ambulatory BP or use of antihypertensive treatment at the 3 month follow-up visit.
Ambulatory BP measurements were performed using the Diasys Integra II device (Novacor, Cedex, France) 3 months after the index stroke to avoid any influence of the recent stroke on the circadian BP rhythm.[Citation18] The cuff was mounted on the non-affected arm, and the patients were instructed to relax their arms when readings were initiated. The device was preset to automatically measure BP and heart rate at 30 min intervals during night-time and 15 min intervals during day-time for a period of 24 h, giving a total of 78 measurements over the 24 h period. Day-time was defined as the interval between 07:00 and 23:00 h and night-time between 23:00 and 07:00 h. If < 70% of the BP recordings were invalid, the ambulatory BP recording was repeated.
Elevated night-time BP was identified as night-time systolic BP ≥ 120 mmHg and/or diastolic BP ≥ 70 mmHg.[Citation19] Non-dipping was considered present if a < 10% reduction in night-time mean from day-time mean BP was present.
Pulse-wave velocity
Carotid–femoral PWV was measured by the same investigator using a SphygmoCor device (AtCor Medical, Sydney, West Ryde, Australia) under standardized laboratory conditions according to the guidelines.[Citation20] Pressure pulse waveforms were obtained transcutaneously from the right common carotid and femoral arteries with simultaneous recording of the electrocardiogram for synchronizing carotid and femoral pulse-wave times, as previously described.[Citation12] Carotid–femoral PWV was calculated as the distance in meters between the two recording sites, divided by transit time in seconds. All PWV values were corrected for mean BP. High for age PWV was taken as PWV higher than age-adjusted normative values, identified by the reference nomogram based upon the Anglo-Cardiff collaborative trial.[Citation21]
Carotid ultrasound
Intima–media thickness (IMT) on the far wall of the common carotid artery on both sides was measured by carotid ultrasound with a 9–3 MHz linear array transducer (iU22; Philips Medical Systems, Bothell, WA, USA) 3–4 days after the index stroke, as previously published.[Citation13] Measurements were performed at four predefined angles, using a Meijers Carotid Arc® (Bio-Imaging Technologies, Leiden, the Netherlands). Carotid plaque was defined as focal maximum IMT > 1.5 mm.[Citation22]
Statistics
Statistical analyses were performed using the IBM SPSS statistical program, version 21 (IBM, Armonk, NY, USA). Data are presented as mean ± standard deviation for continuous variables and as percentages for categorical variables. The population was subdivided according to dipping status into non-dippers and dippers. Comparison of groups was done by the Student’s t test and chi-squared test as appropriate. The variable serum creatinine was skewed and therefore log transformed before being used in univariate and multivariate testing. Univariate correlations of reduction in night-time BP were assessed by Pearson’s correlation coefficient. Univariate covariates of non-dipping and elevated night-time BP were identified by logistic regression analyses and reported as odds ratio (OR) and 95% confidence interval (CI). The most important univariate covariates were included in multivariate logistic regression models. A p value ≤0.05 was considered statistically significant in univariate and multivariate analyses.
Results
The total study population of 268 ischemic stroke patients (mean age 49 ± 10 years) included 68% males, 12% with diabetes mellitus, 44% with hypertension and 61% with hypercholesterolemia (). Among patients with diabetes mellitus, hypertension or hypercholesterolemia, 42%, 56% and 9%, respectively, were on drug treatment. The antihypertensive treatment included angiotensin receptor blockers (48%), beta-blockers (25%) and calcium antagonists (22%).
Table 1. Clinical characteristics of the total study population and groups according to their dipping status.
Non-dipping was found in 38%. Only 12 patients had a reverse dipping pattern (night-time mean BP higher than day-time), and these were included in the non-dipping category. Another seven patients were extreme dippers (night-time BP reduction > 20%), and these were included in the dipping category. These two subtypes of night-time BP pattern were too small to be explored further as individual groups.
Non-dipping
Non-dipping was more common among patients with hypertension, but also found in 35% of normotensive patients. Non-dippers also included more males and patients with diabetes, and were characterized by higher night-time BP, serum creatinine and fasting blood glucose compared to dippers (all p < 0.05) ( and ). Non-dippers also had higher PWV and mean cIMT (both p < 0.05), whereas the prevalence of carotid plaque did not differ between the groups (). In a general linear model, high for age PWV remained more common among non-dippers than dippers after adjustment for age and gender (p = 0.039), but not when adjusted for antihypertensive therapy and 24 h mean BP (p = 0.101). In a similar model, the difference in mean cIMT between non-dippers and dippers became non-significant when adjusted for age and gender. A significant correlation was found between percentage night-time reduction in mean BP and mean cIMT (r = –0.20) and PWV (r = –0.16), and also higher body weight (r = –0.19), waist circumference (r = –0.15), fasting blood glucose (r = –0.20) and glycosylated hemoglobin (HbA1c) (r = –0.22, all p < 0.01). In a multivariate linear regression model, the association between percentage reduction in mean BP from day to night and higher PWV remained significant when adjusted for age and gender (p = 0.046), but the association became non-significant when adjusting for 24 h mean BP and antihypertensive medication. In contrast, the association between the percentage reduction in mean BP from day to night and higher cIMT remained significant after adjustment for age, gender and 24 h mean BP (β = –0.16, p = 0.034).
Table 2. Ambulatory blood pressure (BP) measurements of the total study population and groups according to their dipping status.
In univariate analyses, non-dipping was associated with high for age PWV (OR = 3.00; 95% CI 1.53–5.91, p = 0.001), antihypertensive treatment (OR = 2.09; 95% CI 1.15–3.79, p = 0.016), higher serum creatinine (OR = 1.02; 95% CI 1.01–1.04, p = 0.007) and male gender (OR = 1.86; 95% CI 1.05–3.27, p = 0.032), while no association with 24 h mean BP or age was found (). Non-dipping also tended to be associated with increased mean cIMT (OR = 2.48; 95% CI 0.97–6.30, p = 0.057) ().
Table 3. Univariate predictors of non-dipping and elevated night-time blood pressure (BP).
In multivariate logistic regression analysis, non-dipping was associated with high for age PWV (OR = 2.12; 95% CI 1.07–4.19, p = 0.031) independent of antihypertensive treatment, serum creatinine and male gender ().
Table 4. Independent covariates of non-dipping blood pressure (BP) pattern in multivariate logistic regression analysis.
Elevated night-time blood pressure
Elevated night-time BP was common and found in 80% of non-dippers and in 33% of dippers (). In univariate logistic analyses, male gender (OR = 2.83; 95% CI 1.64–4.87, p < 0.001), obesity (OR = 3.45; 95% CI 1.87–6.36, p < 0.001), diabetes (OR = 2.50; 95% CI 1.05–5.94, p < 0.05) and presence of carotid plaque (OR = 3.01; 95% CI 1.15–7.90, p < 0.05) were particularly associated with the presence of elevated night-time BP (). In univariate linear regression analyses, higher mean night-time BP was associated with higher mean cIMT and PWV (both p < 0.01) (). In multivariate logistic regression analysis, elevated night-time BP was associated with increased cIMT (OR = 3.83; 95% 1.01–14.50, p < 0.05) independent of non-dipping status, male gender, obesity, antihypertensive treatment and high for age PWV ().
Figure 1. Higher night-time mean blood pressure (BP) was associated with (a) higher mean carotid intima–media thickness (IMT) and (b) higher carotid–femoral pulse-wave velocity (PWV).
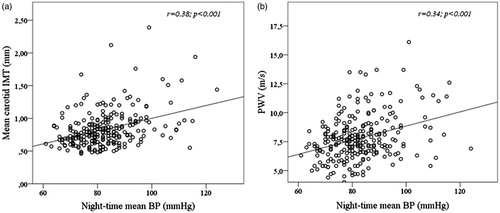
Table 5. Independent covariates of elevated night-time blood pressure (BP) in multivariate logistic regression analysis.
Discussion
This study demonstrates that a non-dipping BP pattern is common among young and middle-aged ischemic stroke patients, found in 38% of the total study population and in 50% of hypertensive patients. Non-dipping was particularly associated with the presence of early arterial stiffening, independent of history of hypertension or reduced renal function. The prevalence of non-dipping in the present study is comparable to a previous report from untreated hypertensive patients at a similar age.[Citation23] Although non-dipping was particularly common in hypertensive young and middle-aged ischemic stroke patients, it was also found in 35% of normotensive patients. Data from the Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events (MAPEC) study previously demonstrated that non-dippers with normal BP carry the same risk for cardiovascular events as dippers with elevated BP independent of absolute BP levels,[Citation24] pointing to the potential prognostic importance of non-dipping also in normotensive ischemic stroke survivors.
As demonstrated by our findings, non-dipping was significantly associated with signs of more generalized arterial damage. High for age PWV, indicating early aortic stiffening, was nearly three-fold more common among non-dippers, and a significantly higher mean carotid IMT, but not the prevalence of carotid plaques, was found in non-dippers, suggesting an association of non-dipping BP pattern with subclinical arterial remodeling. Our findings extend previous reports from a small study of untreated hypertensive patients by Cicek et al., finding non-dippers to have higher arterial stiffness by PWV,[Citation25] as well as a previous report on 900 subjects from the general population in the Oulu Project Elucidating Risk of Atherosclerosis (OPERA) study, finding a non-dipping pattern to be associated with higher mean carotid IMT.[Citation26]
Another key finding of the present study was the association of higher night-time BP with arterial remodeling independent of non-dipping status and antihypertensive treatment. It is well known that night-time BP is an independent and stronger predictor of cardiovascular mortality than day-time BP.[Citation27] In the Korean-ambulatory blood pressure (Kor-ABP) study of 682 hypertensive patients, higher night-time BP rather than non-dipping pattern was associated with the presence of left ventricular hypertrophy.[Citation28] Although elevated night-time BP is often associated with non-dipping, the conditions do not always coincide, as illustrated by the present finding of elevated night-time BP in 80% of non-dippers and 33% of dippers. Syrseloudis et al. demonstrated in a study of hypertensive patients that dippers with elevated night-time BP were characterized by increased central arterial stiffness and subclinical renal damage.[Citation29] In the present study, elevated night-time BP was particularly associated with the presence of male gender, obesity and diabetes, predisposing for subclinical arterial end-organ damage, as indicated by higher PWV and carotid IMT.
Some limitations of our study should be pointed out. First, a systematic assessment of obstructive sleep apnea was not included in the study protocol. Therefore, the influence of sleep apnea on dipping status could not be evaluated. Secondly, circulating biomarkers of atherosclerosis were not measured in our study. Thirdly, the dropout rate was somehow higher than expected in our substudy, but age, gender and the prevalence of cardiovascular risk factors did not differ between the total NOR-SYS population and those included in the present substudy. Finally, any causal relationship between arterial end-organ damage and non-dipping could not be determined in this study owing to the cross-sectional study design.
In conclusion, in the Norwegian Stroke in the Young Study, non-dipping BP pattern and elevated night-time BP were common and associated with high for age arterial stiffness and pre-cerebral arterial remodeling, respectively, independent of clinical cardiovascular risk factors. Our findings point to the value of including ambulatory BP recordings in cardiovascular risk assessment in young and middle-aged stroke survivors.
Acknowledgements
The authors thank the West-Norwegian Regional Health Authority for the financial support and Simon Fougner Hartmanns Family Fund, Oslo, Norway for funding of the SphygmoCor device. We further thank Kristin Modalsli Sand MD, Marina Kokorina MD, and research nurses Liv Himle, Linn Elin Rødal and Toril Synnøve Bjørgo for technical assistance with data collection, registration and patient management.
Disclosure statament
No potential conflict of interest was reported by the authors.
References
- Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26:808–814.
- Sherwood A, Steffen PR, Blumenthal JA, et al. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–118.
- Nakano Y, Oshima T, Ozono R, et al. Non-dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho-vagal nervous activity and progress in retinopathy. Auton Neurosci. 2001;88:181–186.
- Vaile JC, Stallard TJ, al-Ani M, et al. Sleep and blood pressure: spontaneous baroreflex sensitivity in dippers and non-dippers. J Hypertens. 1996;14:1427–1432.
- Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189.
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229.
- Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801.
- Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207.
- Hajjar I, Zhao P, Alsop D, et al. Association of blood pressure elevation and nocturnal dipping with brain atrophy, perfusion and functional measures in stroke and non-stroke individuals. Am J Hypertens. 2010;23:17–23.
- Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857.
- Iwata S, Jin Z, Schwartz JE, et al. Relationship between ambulatory blood pressure and aortic arch atherosclerosis. Atherosclerosis. 2012;221:427–431.
- Saeed S, Waje-Andreassen U, Fromm A, et al. Early vascular aging in young and middle-aged ischemic stroke patients: the Norwegian Stroke in the Young Study. PLoS One. 2014;9(11):e112814.
- Fromm A, Thomassen L, Naess H, et al. The Norwegian Stroke in the Young Study (NOR-SYS): rationale and design. BMC Neurol. 2013;13:89.
- Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Int J Behav Med. 2012;19:403–488.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
- Genuth S, Alberti KG, Bennett P, et al. The expert committee on the diagnosis and classification of diabetes mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–167.
- Mancia G, Fagard R, Narkiewicz K, et al. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2013 ESH/ESC Guidelines for the management of arterial hypertension. Blood Press. 2013;22:193–278.
- Jain S, Namboodri KKN, Kumari S, Prabhakar S. Loss of circadian rhythm of blood pressure following acute stroke. BMC Neurol. 2004;4:1–6.
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
- McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity. the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760.
- Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima–media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th Watching the Risk Symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296.
- Androulakis E, Papageorgiou N, Chatzistamatiou E, et al. Improving the detection of preclinical organ damage in newly diagnosed hypertension: nocturnal hypertension versus non-dipping pattern. J Hum Hypertens. 2015;29:689–695.
- Hermida RC, Ayala DE, Mojón A, Fernández JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level-the “normotensive non-dipper” paradox. Chronobiol Int. 2013;30:87–98.
- Cicek Y, Durakoglugil ME, Kocaman SA, et al. Non-dipping pattern in untreated hypertensive patients is related to increase pulse wave velocity independent of raised nocturnal blood pressure. Blood Press. 2013;22:34–38.
- Vasunta RL, Kesäniemi YA, Ylitalo A, Ukkola O. Nondipping pattern and carotid atherosclerosis in a middle-aged population: OPERA Study. Am J Hypertens. 2012;25:60–66.
- Hansen TW, Li Y, Boggia J, et al. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10.
- Yi JE, Shin J, Ihm SH, et al. Not nondipping but nocturnal blood pressure predicts left ventricular hypertrophy in the essential hypertensive patients: the Korean Ambulatory Blood Pressure multicenter observational study. J Hypertens. 2014;32:1999–2004.
- Syrseloudis D, Tsioufis C, Andrikou I, et al. Association of nighttime hypertension with central arterial stiffness and urinary albumin excretion in dipper hypertensive subjects. Hypertens Res. 2011;34:120–125.
