Abstract
Objectives: A dissociation between behavioural (in-control) and physiological parameters (indicating loss-of-control) is associated with cardiovascular risk in defensive coping (DefS) Africans. We evaluated relationships between DefS, sub-clinical atherosclerosis, low-grade inflammation and hypercoagulation in a bi-ethnic sex cohort. Methods: Black (Africans) and white Africans (Caucasians) (n = 375; aged 44.6 ± 9.7 years) were included. Ambulatory BP, vascular structure (left carotid cross-sectional wall area (L-CSWA) and plaque counts), and markers of coagulation and inflammation were quantified. Ethnicity/coping style interaction was revealed only in DefS participants. Results: A hypertensive state, less plaque, low-grade inflammation, and hypercoagulation were more prevalent in DefS Africans (27–84%) than DefS Caucasians (18–41%). Regression analyses demonstrated associations between L-CSWA and 24 hour systolic BP (R2 = 0.38; β = 0.78; p < 0.05) in DefS African men but not in DefS African women or Caucasians. No associations between L-CSWA and coagulation markers were evident. Conclusion: Novel findings revealed hypercoagulation, low-grade inflammation and hyperkinetic BP (physiological loss-of-control responses) in DefS African men. Coupled to a self-reported in-control DefS behavioural profile, this reflects dissociation between behaviour and physiology. It may explain changes in vascular structure, increasing cerebrovascular disease risk in a state of hyper-vigilant coping.
Introduction
An urban environment, as a psychosocial stressor, contributes to behavioural lifestyle risk factors, including an unhealthy diet, substance abuse and lack of exercise.[Citation1,Citation2] Styles of coping with psychosocial stress have been related to lifestyle diseases such as hypertension, stroke risk and structural endothelial changes.[Citation3] Malan et al. have demonstrated that Africans attempting to adapt to an urban-dwelling lifestyle utilize defensive coping (DefS) and are at greater risk of hypertension and related cardiometabolic pathology than rural DefS Africans or urban DefS Caucasians.[Citation4–6]
The DefS response to psychological stress is a behavioural and physiological “being-in-control” response, i.e. a problem-focused coping style which involves actively trying to find solutions to problems.[Citation7] DefS is considered to be health promoting and is associated with a mainly β-adrenergic central cardiac response.[Citation8,Citation9] Emotional “loss-of-control” has mainly been associated with an α-adrenergic profile and a depressive state.[Citation10,Citation11] However, dissociation between DefS behaviour and physiology was apparent in urban-dwelling Africans.[Citation5,Citation12] DefS Africans reported behavioural control, but a contrasting physiological loss-of-control response with a predominantly α-adrenergic vascular response.[Citation4–6]
If a coping strategy fails, stress pathology may develop because of overarousal of the sympathetic nervous system, thereby increasing the risk of related cardiovascular diseases).[Citation13,Citation14] The specific mechanisms through which psychological stress contributes to structural endothelial changes, apart from a low-grade inflammatory process, are unclear. It has been proposed that psychological stress adversely affects the haemostatic system, with an imbalance between coagulation (thrombus formation) and fibrinolysis (thrombus degradation).[Citation15,Citation16] Thus, dissociative coping strategies in stressful life situations may indirectly “mask” or exaggerate the impact of stress on haemostasis. It has previously been shown that vascular α-adrenergic, and not β-adrenergic responses, were associated with increased plasma D-dimer levels, indicating a state of hypercoagulation.[Citation17] In several studies, D-dimer and fibrinogen demonstrated a strong correlation with psychological stress,[Citation17–19] and D-dimer may play a direct role in the progression of structural vascular disease as it promotes the expression of several genes related to atherogenesis.[Citation20]
To our knowledge, the relationship between coping, inflammation–coagulation profiles and subclinical atherosclerosis risk has not been reported in either Africans or Caucasians from South Africa. The Sympathetic activity and Ambulatory Blood Pressure in Africans (SABPA) study is ideally suited for this assessment, as seasonal changes that could disturb haemostasis have been avoided. We therefore first aimed to determine whether dissociation between behaviour and physiological coping responses would be evident. Secondly, we aimed to ascertain whether the relationship between subclinical atherosclerosis, coagulation and inflammation would support vulnerability and increased risk of future cardiovascular disease.
Material and methods
The SABPA study is a target population study which was conducted in 2008 and 2009 in the Dr Kenneth Kaunda Education district, North-West Province, South Africa. The study sample included 409 ethnic–gender-grouped teachers to ensure homogeneity regarding socio-economic status and working environment, although participants were from diverse cultural backgrounds. Eligible participants between 20 and 62 years of age were invited to participate. Participants were informed about the study protocol before being recruited, after which willing participants signed an informed consent form. The study was approved by the Ethics Review Board of North-West University (Potchefstroom Campus: NWU-0003607S6), and procedures complied with the terms and guidelines of the Declaration of Helsinki.[Citation21]
Exclusion criteria for the SABPA study were the use of α- or β-blockers, blood donation or vaccinations less than 3 months before data collection, and psychotropic substance dependence or abuse. The SABPA study included 202 male and 207 female participants. We excluded 13 men and seven women who tested positive for the human immunodeficiency virus (HIV), seven men and four women who were clinically diagnosed diabetics, and a man and woman each with tympanic temperatures above 37.5 °C. After exclusion, 170 Africans (81 men and 89 women) and 206 Caucasians (106 women and 100 men) remained. No participant was on anticoagulant drugs.
Ambulatory blood pressure measurements
Clinical assessments were obtained over a 48 h period. On Monday to Thursday mornings, each participant was fitted with an ambulatory blood pressure (BP) monitor (with suitable cuff-size on the non-dominant arm), an electrocardiogram (ECG) device and an accelerometer (Cardiotens CE120®; Meditech, Budapest, Hungary; and Actical, Montreal, Quebec). The Cardiotens CE120 was validated by the British Hypertension Society in 2003, and provided oscillometric 24 h ambulatory blood pressure monitoring (ABPM) and two-lead ECG measurements. The device measured BP every 30 min between 08:00 and 22:00 h and every 60 min between 22:00 and 06:00 h.[Citation22] The successful mean inflation rate was 79.18%. Hypertension was classified as an average of ≥ 130 mmHg systolic blood pressure (SBP) and/or ≥ 80 mmHg diastolic blood pressure (DBP) over 24 h.[Citation23] Participants proceeded with their normal daily activities and were asked to record periods of stress, headache, fainting, nausea, palpitations and visual disturbances on their ambulatory diary card. Data were analysed using CardioVisions 1.15.2 Personal Edition (Meditech, Budapest, Hungary).
At approximately 16:30 h on day 1, participants were transported to North-West University’s Metabolic Unit Research Facility. Here, they were familiarized with the experimental set-up and completed a psychosocial battery under the supervision of registered clinical psychologists. A standardized dinner was served and participants were asked go to bed at 22:00 h while fasting overnight. At 06:00 h on day 2, after the last BP reading was obtained, the Cardiotens and accelerometer were removed and anthropometric measurements were conducted. Participants rested in a semi-recumbent position for a period of 15–20 min, whereupon resting venous blood sampling, followed by ultrasound scanning, was performed. Information regarding each participant’s general health, socio-demographic and medical history was obtained.
Assessment of lifestyle risk factors
Participants’ physical activity in kcal/day was measured using the Actical® omnidirectional accelerometer. This device measured movement at 15 s intervals, and data were converted into 1 s intervals for analysis. γ-Glutamyltransferase (γ-GT) was measured as a marker for alcohol abuse.[Citation24] Cotinine was measured to determine participants’ smoking status, with subjects regarded as smokers if cotinine values exceeded 14.99 ng/ml.[Citation25] Anthropometric measurements were performed in triplicate by level II registered biokineticists using standardized methods (Precision Health Scale; A&D Company, Tokyo, Japan; and Invicta Stadiometer IP 1465; Invicta, London, UK). Body surface area in m2 was determined using the Mosteller formula.[Citation26] Interobserver and intraobserver variability was less than 10%.
Ultrasonography
Left carotid intima–media thickness of the far wall (L-CIMTf) of a 10 mm segment of the common carotid artery (CCA), 10 mm distal to the carotid bulb, was used to determine early subclinical structural vascular changes ().[Citation27] The Sonosite Micromaxx ultrasound system (SonoSite, Bothell, WA, USA) was used to perform ultrasonography with a 6–13 MHz linear array transducer according to the Meijer protocol, where the carotid arc was used to image the artery at five prespecified angles in steps of 30 degrees (from 60 degrees to 180 degrees on the right carotid artery and from 300 degrees to 180 degrees on the left carotid artery).[Citation28] All images were measured in end-diastole at the top of the R wave of the electrocardiogram. Ultrasonographs were digitally analysed with Artery Measurement Systems automated software (AMS) II v1.139 (Gothenburg, Sweden). This method has been validated for use of carotid intima–media thickness (CIMT) to identify the risk of subclinical vascular disease.[Citation27] Left cross-sectional wall area (L-CSWA) was calculated as L-CSWA = π(d/2 + L-CIMTf)2 – π(d/2)2, with d as the luminal diameter of the far wall of the left CCA.[Citation7,Citation29]
Figure 1. B-mode ultrasound image of the left carotid artery, indicating the carotid bulb (on the left) and common carotid artery (on the right). The region of interest, where intima–media thickness was measured, is a 10 mm segment of the common carotid artery located 10 mm distal to the carotid bulb. All measurements were taken at full arterial dilation (R-wave). This image was obtained from a defensive coping African male with a mean carotid intima–media thickness of 0.77 mm and a plaque score of 2, indicating a small and isolated thickening.
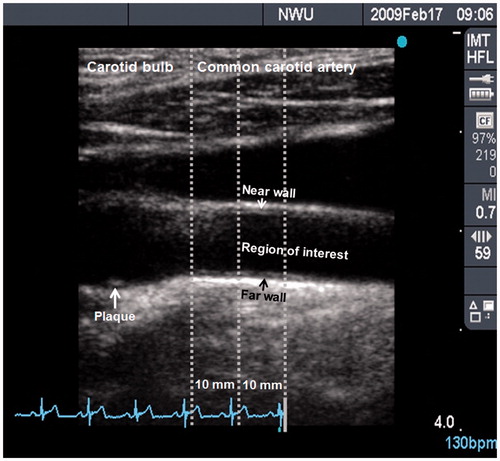
Plaque scores were obtained from the near and far walls of the left and right CCA, bifurcation and internal carotid artery. Images were read by four readers who completed a uniform training programme which ensured standardized settings across reading stations and core laboratories. If a plaque was present, ultrasound images were recorded along with information on the number and location of plaques. A plaque was defined as a focal structure that encroaches into the arterial lumen for at least 0.5 mm or 50% of the surrounding CIMT value, or demonstrates a thickness of at least 1.5 mm as measured from the media–adventitia interface to the intima–lumen interface.[Citation30] For qualitative assessment, plaques were graded as follows: 1, none (complete absence, CIMT thickening may be observed); 2, minimal (small isolated thickening); 3, moderate (visible plaque, causing a modest degree diameter reduction); 4, severe (significant plaque, visualized on several different scan projections, causing a clear reduction in diameter); or 5, occlusion [Citation30]
Coping Strategy Indicator
Coping responses were assessed using the Coping Strategy Indicator (CSI),[Citation7] which assesses defensive active problem solving (DefS) and emotional avoidance strategies as well as seeking social support when faced with difficult situations. The 33 items on the questionnaire form three subscales (DefS/in-control, avoidance/loss of control, seeking social support) with 11 items each. Items are rated with a three-point Likert scale: 3 = a lot, 2 = a little and 3 = not at all. Respondents are asked to remember a recent stressful event and to consider the way they coped with it. Responses that denote problem solving, acceptance of stressors as reality, actively striving to eliminate stressors and being-in-control imply a defensive coping response which is recognized as a health promoter;[Citation9] seeking social support implies an active process which is focused on obtaining advice and comfort during stressful times; lastly, emotional avoidance implies a defeat response, with physical as well as psychological withdrawal. Above-mean coping responses have been determined as ≥ 26 for DefS, ≥ 23 for social support and ≥ 19 for avoidance.[Citation7] The CSI has been proved to be applicable in the African context. Calculated α-Cronbach coefficients of 0.83 for DefS, 0.84 for seeking social support and 0.69 for emotional avoidance confirmed the reliability of coping responses for our sample.
Biochemical sampling and analyses
Fasting resting blood samples were obtained before 09:00 h by a registered nurse from the brachial vein branches using a sterile winged infusion set. Fibrinogen and D-dimer were selected as coagulation markers because of their strong correlation with psychological stress.[Citation17] Fibrinogen and D-dimer citrate samples were measured using viscosity- and immuno-based clotting methods, respectively (STA Compact; STAGO Diagnostic, Roche, Meylan, France). Serum γ-GT was measured using a high-sensitivity enzyme rate method, resting sodium fluoride glucose and serum cholesterol were measured using the timed end-point method, and serum C-reactive protein (CRP) as a marker for inflammation was measured using highly sensitive turbidimetry (Unicel DXC 800; Beckman and Coulter, Krefeld, Germany). Serum cotinine was measured using the homogeneous immunoassay method (Modular Roche Automized; Roche, Basel, Switzerland; and Konelab™ 20I Sequential Multiple Analyser Computer; ThermoScientific, Vantaa, Finland). Glycosylated haemoglobin (HbA1c), a reflection of average capillary glucose for the preceding 2–3 months, was measured using a turbidimetric inhibition immunoassay (Cobas Integra 400plus; Roche, Basel, Switzerland). EDTA plasma HIV status was measured using the First Response kit (RPM Plus, Chicago, USA) and the Pareekshak test (Bhat Biotech, Bangalore, India).
Statistical analyses
Data were analysed using Statistica version 12.0 (StatSoft, Tulsa, USA, 2013). Distribution of data was tested using the Kolmogorov–Smirnov test for normality. γ-GT and cotinine were logarithmically transformed to obtain a Gaussian distribution, and extreme outliers for all variables were manually removed. Student’s t tests were used to describe the population by ethnic status and to determine significant a priori confounders (age, body surface area, physical activity, log γ-GT and log cotinine) (Citation23] Chi-squared tests were used to determine proportions and plaque score counts. Established cardiometabolic risk cut-offs were calculated as the percentage of population above the cut-off for fibrinogen (≥ 3.5 g/l), D-dimer (≥ 300 ng/ml) and CRP (≥ 3.0 mg/l).[Citation31] Three-way analysis of covariance (ANCOVA), independent of a priori covariates, was used to determine significant interactions between main effects (Ethnicity × Gender × Coping) for inflammation, coagulation and cardiometabolic markers. Subsequently, one-way ANCOVAs were used to determine differences in ethnic–gender–coping groups independent of a priori covariates. Significance was determined using Benjamini–Hochberg correction for false discovery with a chosen false discovery rate of 0.1;[Citation32] the significance level was hereafter set at p < 0.05. Multiple linear regression analyses were computed. Forward stepwise regression analyses determined associations between structural endothelial dysfunction, inflammation, coagulation and other cardiometabolic markers. The dependent variables comprised fibrinogen, D-dimer, L-CIMTf and L-CSWA. Independent variables for the fibrinogen and D-dimer models were BP, HbA1c and a priori covariates. For the L-CIMTf and L-CSWA models, the independent variables were total cholesterol, CRP, BP, HbA1c and a priori covariates. These models determined whether independent associations existed between coagulation, inflammation and endothelial dysfunction in (i) separate ethnic–gender groups comprising all participants, and (ii) separate ethnic–gender groups comprising only DefS participants. Significant values were denoted as p ≤ 0.05.
Results
presents the baseline characteristics of the two ethnic groups. Caucasians showed significantly higher body surface area (p ≤ 0.05) and 24 h total energy expenditure (physical activity) than Africans. The Africans demonstrated a greater cardiovascular risk profile, with higher γ-GT, a higher proportion of smokers, low-grade inflammation, hypercoagulation and mean hypertensive profiles. Mean fibrinogen and D-dimer levels exceeded the cut-off point in the Africans (> 3.0 g/l and >300 ng/ml, respectively). In the Caucasians, fibrinogen levels met the cut-off criteria at 3.0 g/l. Mean DefS scores tended to be above the mean (≥26) for both groups and were similar in value. Africans used more social support and less avoidance than Caucasians. Univariate tests for significance demonstrated interactions of L-CIMTf with gender (F[0.202]) = 8.53, p < 0.005) and L-CIMTf with race × DefS (F[0.153]) = 6.47, p < 0.01). Neither avoidance nor social support coping strategies showed significant interactions pertaining to any of the coagulation or structural endothelial risk markers. This necessitated the selection of participants with above mean DefS scores (≥ 26), within each ethnic group, and their division into separate genders ( and ; and ).
Figure 2. Prevalence of exceeded cut-off values for fibrinogen, D-dimer and C-reactive protein for: (a) all men, defensive active coping (DefS) African and Caucasian men, and (b) all women, DefS African and Caucasian women. An asterisk (*) denotes p ≤ 0.05 vs the DefS African gender.
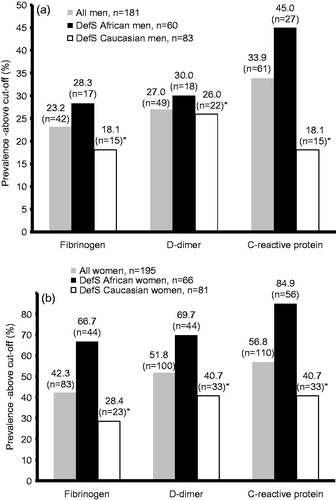
Figure 3. Carotid artery plaque scores (counts) of all men and women (Total group), total defensive coping (DefS) bi-gender cohort, and DefS African and Caucasian men and women from the SABPA study.
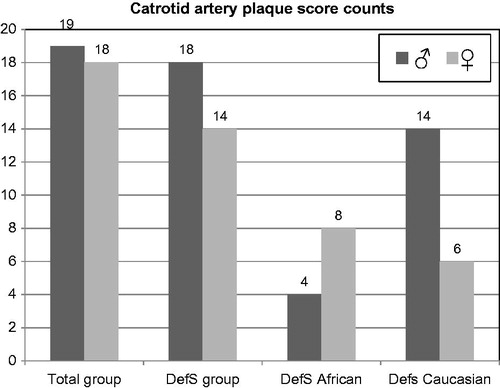
Table 1. Baseline characteristics by ethnic status (mean ± SD).
Table 2. Adjusted comparisons between defensive coping (DefS) Africans and Caucasians.
Table 3. Independent associations between coagulation, structural endothelium and cardiovascular disease markers in all African men and defensive coping (DefS) African men.
A similar trend as observed for the total African group in was evident in for DefS African men and women when compared to their Caucasian counterparts. Independent of a priori covariates, risk markers showed that D-dimer levels in the African gender groups were higher than in the Caucasian gender groups, but only the African females’ mean D-dimer level was above the cut-off point. Furthermore, coagulation markers were higher in the females compared to their male counterparts. Cardiovascular risk markers exceeded established cut-off values in the DefS Africans, i.e. CRP (> 3.0 mg/l), pre-diabetic state (HbA1c > 5.7%) and 24 h ABPM hypertension (≥ 130 mmHg and/or ≥ 80 mmHg).[Citation23,Citation31]
shows the prevalence of fibrinogen, D-dimer and CRP exceeding the cut-off values for the total group and DefS men, with the prevalence in DefS African women being higher in all three instances. In , more plaques were evident in the DefS Caucasian men, coupled to higher cholesterol and statin medication usage. The DefS men accounted for all but one of the plaques present in the study group (total group = 19 and DefS men = 18).
Forward stepwise regression analyses in present the adjusted associations between dependent variable, structural endothelium and independent predictors, namely coagulation and cardiometabolic risk markers, in all African men and in DefS African men. No independent associations between the hypercoagulation and structural endothelial markers were shown in any group. Systolic ABPM was strongly associated with structural endothelial changes (L-CIMTf and L-CSWA) in all African men and particularly in DefS African men. D-dimer models showed no significant independent association in any group.
Discussion
We aimed to assess whether dissociation between DefS behaviour and physiological responses is evident, and whether the relationship between subclinical atherosclerosis, coagulation and inflammation would support vulnerability and increased risk of cardiovascular disease. The main findings revealed a self-reported behavioural in-control (β-adrenergic) pattern but a physiological loss-of-control (α-adrenergic) pattern in the most vulnerable group,[Citation5] i.e. the DefS African men. Their mean hypertensive ABPM SBP values predicted structural endothelial changes in linear regression models. Despite coagulation markers exceeding cut-off values, no associations existed for these markers with structural endothelial changes. This underscores the disparities between studies on whether coagulation, via fibrinogen synthesis, is increased during psychological stress.[Citation16] Several other studies, however, reported that D-dimer, a marker of fibrin turnover, is increased in psychological stress independent of cardiovascular disease status.[Citation15,Citation19] In our participants, fibrinogen levels of all DefS participants exceeded the accepted cut-off point, except for Caucasian men, who were in the upper-normal range. The higher fibrinogen levels in the women may be partially explained by their post-menopausal status.[Citation33] A hypercoagulable state is likely to contribute to atherosclerosis,[Citation34] but in our study no significant direct association between either fibrinogen or D-dimer and the structural vascular disease markers L-CIMTf and L-CSWA was detected. In a meta-analysis concerning the relation of various biochemical markers to CIMT, only fibrinogen and CRP showed a strong correlation with CIMT.[Citation34] We could not replicate any of these findings.
L-CIMTf and L-CSWA showed strong independent correlations with 24 h SBP in the DefS African men but not in any other group. This suggests that elevated SBP, as a hyperkinetic driving force, is strongly related to arterial remodelling in DefS African men only, increasing the risk of arterial wall stiffening. Reference values for CCA intima–media thickness have not yet been firmly established for Africans, but reference values for European Caucasians indicate that our participants’ mean L-CIMTf falls below the cut-off point for cardiovascular risk (< 0.81 mm).[Citation35]
In the DefS African cohort, hypercoagulation was demonstrated by the augmented D-dimer and fibrinogen levels, indicating higher fibrin turnover, which corresponds with previous research.[Citation19] These responses support the notion of dissociation between behaviour and physiology, where a physiological loss-of-control DefS style may support predominant α-adrenergic activity despite a behavioural β-adrenergic in-control defensive coping response.[Citation4,Citation6,Citation13] Stress-induced increases in D-dimer and the response patterns of the α-receptor agonist noradrenaline (norepinephrine) are related.[Citation36] Therefore, we suggest that this vasoconstrictive pattern may further enforce an increase in ambulatory BP values and the risk of coronary artery disease.[Citation37] Noradrenaline’s action on the α-adrenoceptors constitutes a trophic stimulus to cardiac and vascular smooth muscle hypertrophy. In other substudies from the SABPA cohort, hypertensive DefS African men had increased left ventricular hypertrophy values, α-adrenergic BP responses, ischaemia, sympathovagal disturbances [Citation6] and attenuated noradrenaline metabolite responses.[Citation13] These responses have been associated with sympathetic hyperactivity,[Citation13] indicating down-regulated cardiac noradrenaline spillover, which predisposes patients to sudden cardiac death.[Citation38] Ultimately, autonomic exhaustion (neural fatigue) and sympatho-adrenal–medullary system desensitization may therefore disturb structural endothelial integrity if a coping strategy fails. We carefully suggest that dissociation in the African men may be defined as “coping failure” or a desensitized neural system during hypervigilant coping.
Vulnerability in the African men can further increase as D-dimer and its relationship to α-adrenergic response patterns may promote the expression of several genes related to atherogenesis. Affected processes include molecule adhesion to the vascular wall, lipid transport and metabolism, proliferation and cell growth, stress responses, gene transcription regulation and extracellular molecule regulation.[Citation20] Augmented D-dimer values acted in tandem with low-grade inflammation and a mean hypertensive status, exposing a cardiovascular vulnerability in African men when in a state of chronic defensiveness.[Citation6,Citation12] Inevitably, they may be at a higher risk of developing subclinical atherosclerosis than their Caucasian counterparts, despite the African men’s lower cholesterol values. Most intriguing is the fact that in total, 19 plaque score counts were evident in the total male group. Of these plaque scores, 18 were found in DefS men (four in DefS Africans and 14 in Caucasian men). The higher plaque score counts in the DefS Caucasian men are supported by their higher cholesterol despite their higher hypercholesterolaemia medication usage. This may underpin different underlying mechanisms for cardiovascular risk in these ethnic groups. Conversely, more plaques were evident in the African women than in the Caucasian women. This may partially result from their significantly higher fibrinogen, D-dimer and CRP values, which may be influenced by the higher oestradiol and hormone replacement medication usage of the African women.[Citation20,Citation34]
The dissociation between DefS behaviour and physiology was only evident in the Africans, implying more psychological distress [Citation39] and explaining the at-risk responses. Reasons for higher defensive responses may include a demanding urban environment [Citation39] where cultural changes disrupt collectivistic cohesiveness, impairing adaptability and ultimately increasing allostatic load.[Citation2,Citation6,Citation40] These findings reinforce the need for teaching coping strategies at an early age. Acquiring adaptive coping skills at a tender age may reduce situational stress at that age and later in life.[Citation41,Citation42]
Our study has its limitations. Replication in larger samples may strengthen our findings. As this study is novel in South Africa and the cohort was restricted to two ethnicities, we cannot generalize our results to other South African populations and ethnicities. In particular, similar well-controlled studies in other urban regions of South Africa, on these and other ethnicities, could further our knowledge on the important role of coping failure in cardiovascular physiology. Our study was cross-sectional and more reliable results might be gained by following the participants over time with repeated measurements. However, subjects were chosen to reflect homogeneity, rigorous exclusion criteria were applied, gold standard measures were obtained, and participants were assessed within a well-controlled environment while standardized procedures were followed.
In conclusion, our study shows that both African and DefS Caucasian men exhibited a state of hypercoagulability, as evidenced by elevated D-dimer values. These values were enhanced in DefS Africans who additionally demonstrated low-grade inflammation and hypertensive BP status. The hypercoagulation status in DefS African men increases cardiovascular morbidity and risk of coronary artery syndrome. Our findings support the notion of dissociation between behaviour and physiology in DefS African men. They reported behavioural control, although they exhibited a loss of physiological control or coping failure. Low-grade inflammation in DefS African males in tandem with hypercoagulation and apparent hyperkinetic BP may manifest in subclinical atherosclerosis and increase their cerebrovascular disease risk.
Acknowledgements
The authors would like to thank the participants for their involvement in the study. We would also like to thank Dr Szabolcs Péter, Sr Chrissie Lessing and Mrs Tina Scholtz for their technical assistance and support.
Declaration statement
No potential conflict of interest was reported by the authors.
LM accepts responsibility for the integrity of the data. JDS takes full responsibility for the accuracy of the data analyses. All authors contributed to the design of the study, and the drafting and critical revision of this manuscript.
Funding information
This research was partly funded by The Metabolic Syndrome Institute, France; the Medical Research Council, National Research Foundation, Roche Diagnostics, North-West University, and North West Department of Education, South Africa. The opinions expressed in this article are not necessarily those of the National Research Foundation. The funding bodies were not involved in the study design and management, data collection and analyses, or preparation of this manuscript.
References
- Roberts C, Troop N, Connan F, et al. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity (Silver Spring, Md). 2007;15:3045–3055.
- Rozanski A, Kubzansky LD. Psychologic functioning and physical health: a paradigm of flexibility. Psychosom Med. 2005;67(Suppl 1):S47–53.
- Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. J Pers Soc Psychol. 1986;50:571–579.
- Malan L, Hamer M, Reimann M, et al. Defensive coping, urbanization, and neuroendocrine function in Black Africans: the THUSA study. Psychophysiology. 2012;49:807–814.
- Malan L, Hamer M, Schlaich MP, et al. Facilitated defensive coping, silent ischaemia and ECG left-ventricular hypertrophy: the SABPA study. J Hypertens. 2012;30:543–550.
- Malan L, Hamer M, Schlaich MP, et al. Defensive coping facilitates higher blood pressure and early sub-clinical structural vascular disease via alterations in heart rate variability: the SABPA study. Atherosclerosis. 2013;227:391–397.
- Amirkhan JH. A factor analytically derived measure of coping: the Coping Strategy Indicator. J Pers Soc Psychol. 1990;59:1066–1074.
- Light KC. Psychosocial precursors of hypertension: experimental evidence. Circulation. 1987;76:I67–176.
- van Rhenen W, Schaufeli WB, van Dijk FJ, Blonk RW. Coping and sickness absence. Int Arch Occup Environ Health. 2008;81:461–472.
- Garcia-Leon A, Reyes del Paso GA, Robles H, Vila J. Relative effects of harassment, frustration, and task characteristics on cardiovascular reactivity. Int J Psychophysiol. 2003;47:159–173.
- Thomas KS, Nelesen RA, Ziegler MG, et al. Job strain, ethnicity, and sympathetic nervous system activity. Hypertension. 2004;44:891–896.
- Malan L, Malan NT, Wissing MP, Seedat YK. Coping with urbanization: a cardiometabolic risk? The THUSA study. Biol Psychol. 2008;79:323–328.
- de Kock A, Malan L, Hamer M, Malan NT. Defensive coping and subclinical vascular disease risk – associations with autonomic exhaustion in Africans and Caucasians: the SABPA study. Atherosclerosis. 2012;225:438–443.
- Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246.
- Wirtz PH, Ehlert U, Emini L, et al. Anticipatory cognitive stress appraisal and the acute procoagulant stress response in men. Psychosom Med. 2006;68:851–858.
- Bennett PC, Lane DA, Lip GY. Vital exhaustion and cardiovascular disease: are circulating fibrinogen and D-dimer levels a plausible link? Stress. 2008;11: 247–249.
- Austin AW, Wissmann T, von Kanel R. Stress and hemostasis: an update. Semin Thromb Hemost. 2013;39:902–912.
- Mausbach BT, Ancoli-Israel S, von Känel R, et al. Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep. 2006;29:1347–1352.
- von Känel R, Dimsdale JE. Fibrin D-dimer: a marker of psychosocial distress and its implications for research in stress-related coronary artery disease. Clin Cardiol. 2003;26:164–168.
- Zhou D, Yang PY, Zhou B, Rui YC. Fibrin D-dimer fragments enhance inflammatory responses in macrophages: role in advancing atherosclerosis. Clin Exp Pharmacol Physiol. 2007;34:185–190.
- World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403–405.
- Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26:808–814.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2013;34:2159–2219.
- Hastedt M, Buchner M, Rothe M, et al. Detecting alcohol abuse: traditional blood alcohol markers compared to ethyl glucuronide (EtG) and fatty acid ethyl esters (FAEEs) measurement in hair. Forensic Sci Med Pathol. 2013;9:471–477.
- Jarvis M, Tunstall-Pedoe H, Feyerabend C, et al. Biochemical markers of smoke absorption and self reported exposure to passive smoking. J Epidemiol Community Health. 1984;38:335–339.
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima–media thickness task force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111.
- Liang YL, Teede H, Kotsopoulos D, et al. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci (Lond). 1998;95:669–679.
- Roman MJ, Pickering TG, Schwartz JE, et al. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. J Am Coll Cardiol. 1996;28:751–756.
- Hennerici MG, Bots ML, Ford I, et al. Rationale, design and population baseline characteristics of the PERFORM vascular project: an ancillary study of the Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack (PERFORM) trial. Cardiovasc Drugs Ther. 2010;24:175–180.
- Porter RS. (Ed). The Merck manual of diagnosis and therapy, 19th ed. Merck Research Laboratories, Whitehouse Station, NJ: Wiley; 2011.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
- Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96:711–729.
- Baldassarre D, De Jong A, Amato M, et al. Carotid intima–media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med. 2008;40:21–44.
- Touboul PJ, Labreuche J, Vicaut E, et al. Country-based reference values and impact of cardiovascular risk factors on carotid intima–media thickness in a French population: the “Paroi Arterielle et Risque Cardio-Vasculaire” (PARC) study. Cerebrovasc Dis. 2009;27:361–367.
- von Känel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65:357–369.
- Preckel D, von Känel R. Regulation of hemostasis by the sympathetic nervous system: any contribution to coronary artery disease? Heart Drug. 2004;4:123–130.
- Schlaich MP, Kaye DM, Lambert E, et al. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565.
- Hamer M, Malan L. Psychophysiological risk markers of cardiovascular disease. Neurosci Biobehav Rev. 2010;35:76–83.
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185.
- Diehl M, Coyle N, Labouvie-Vief G. Age and sex differences in strategies of coping and defense across the life span. Psychol Aging. 1996;11:127–139.
- Frydenberg E, Lewis R. Teaching coping to adolescents: when and to whom? Am Educ Res J. 2000;37:727–745.
