Abstract
Background. Glomerular filtration rate (GFR) measured through technetium-99m diethyl triamine penta-acetic acid (Tc99mDTPA) renal scintigraphy (rsGFR) was compared with that estimated (eGFR) from 24-h creatinine clearance (CrCl) and, using both the Cockcroft–Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulas, in a population of hypertensive subjects (HTs) with normal serum creatinine (SCr) levels. Patients and methods. In 200 normoalbuminuric (<30 mg/24 h) HTs 55–75 years old, without diabetes and history of coronary and cerebrovascular diseases, Pearson's correlation assess the relationship between rsGFR and eGFR. The Bland–Altman method was used to assess the agreement between rsGFR and eGFR, separately in subjects with low (<60 ml/min/1.73 m2) and normal (≥60 ml/min/1.73 m2) rsGFR. The span between −1.96 and +1.96 standard deviations of mean difference (bias) was calculated and used for this purpose. Results. In 76 subjects, an unknown low renal function was found by Tc99mDTPA renal scintigraphy. In subjects with normal rsGFR the Bland–Altman analysis showed that the smallest span between rsGFR and eGFR was evident for ClCr values (26.0 ml/min/1.73 m2), whereas higher values were detected with the CG and MDRD formulas (41.0 and 40.4 ml/min/1.73 m2, respectively). The same results were observed for low rsGFR, where a smaller span was found for ClCr (21.2 ml/min/1.73 m2), whereas CG and MDRD methods gave greater results (30.4 and 31.8 ml/min/1.73 m2 respectively); no differences were found between genders. The degree of agreement for eGFR estimated with the CG and MDRD formulas was wider than that derived from ClCr, reflecting a greater between-methods variability and a considerable discrepancy of rsGFR values in the former than in the latter. Conclusions. In HTs with normal SCr values, Tc99mDTPA renal scintigraphy discovered un known renal organ damage in 38% of cases. If this methodology is not available, ClCr measurement should be preferred to estimate GFR whereas CG and MDRD formulas are of limited efficacy.
Introduction
Glomerular filtration rate (GFR) is one of the commonly used indexes for the early detection of chronic kidney disease (CKD), a condition recognized to be an emergent risk factor for overall and cardiovascular (CV) mortality (Citation1). This is particularly evident for hypertensive subjects (HTs) in which high blood pressure (BP) levels determine a progressive impairment of renal function and an increased risk of CKD (Citation2).
In clinical practice, indirect methods to approximate GFR are used and the most frequently employed is serum creatinine concentration (SCr). However, the role of SCr as an indicator of renal function is limited, as its level is influenced by many non-renal factors including muscle mass, age and the method of measurement (Citation3–5). Creatinine clearance (CrCl) determination through 24-h urine collection is also used to estimate renal function but the reliability of this method is very much dependent on accurate and complete urine collection (Citation6,Citation7). Furthermore, earlier studies focused on SCr and ClCr measurement as markers of GFR showed that SCr usually does not increase until GFR has decreased by 50% or more, and many subjects with normal SCr levels frequently have lower GFR (Citation8,Citation9). In order to overcome these limitations, creatinine-based estimating equations has been introduced in clinical practice as a rapid method for GFR evaluation (Citation10). The Cockcroft & Gault equation (CG) (Citation11) and the abbreviated Modification of Diet in Renal Disease (MDRD) formula (Citation12) were the most widely used in clinical practice, becoming a powerful screening tool for early detection of CKD. However several analyses have demonstrated limitations in the accuracy and applicability of these formulas for the renal function estimation, particularly in subjects with normal SCr levels (Citation13,Citation14). Fortunately, an accurate evaluation of GFR independently of SCr levels is available by measuring the clearance of radiolabelled isotopes such as technetium-99m diethyl triamine penta-acetic acid (Tc99mDTPA) during renal scintigraphy, which is considered the gold standard method for estimating GFR (Citation15). Nevertheless, it is common opinion that Tc99mDTPA renal scintigraphy is invasive and expensive, and not readily available in all clinical settings (Citation16,Citation17). For these reasons, the use of methods rather than another to estimate GFR could influence the early detection of the preclinical organ renal damage, a condition that strongly increases the global CV risk of HTs (Citation18).
The aim of this study was to evaluate the interchangeability of methods for assessment of GFR estimated by CrCl, CG and MDRD formulas against the GFR measured by Tc99mDTPA renal scintigraphy, in a population of hypertensive, non-diabetic and normoalbuminuric subjects with normal SCr levels.
Material and methods
Subjects and study design
This study enrolled 200 hypertensive subjects (94 males and 104 females) aged between 55 and 75 years, referred to our Hypertension Centre from September 2008 to October 2009. Exclusion criteria were severe hypertension (office systolic BP ≥180 mmHg or diastolic BP ≥110 mmHg), secondary hypertension, diabetes mellitus, presence of neoplastic or hepatic disease, chronic heart failure, a positive history or clinical signs of ischaemic heart disease, severe obesity (defined as body weight >150% of the ideal body weight), and/or disabling diseases such as dementia or inability to co-operate. The presence of a Hypertension Centre represents an important landmark for general practitioners as the decision about the management of HTs should not be based on the level of BP alone, but also on the presence of other risk factors and pre-clinical target organ damage (TOD), this latter difficult to evaluate in primary care.
Fasting SCr (in mg/dl) and urinary creatinine were measured using the alkaline picrate-kinetic method of Jaffè (Citation19) by an auto-analyser (Hitachi Modular P, Roche diagnostic, USA). Albuminuria was measured using the turbidimetric method (Cobas Mira Plus, Roche, Montclair, NJ) in a specimen of urine collected for 24 h. All subjects had normal SCr levels, i.e. (<1.2 mg/dl in men and <0.9 mg/dl in women) and were normoalbuminuric (<30 mg/24 h).
In all subjects, GFR was directly measured by renal scintigraphy (RS) and estimated by different methods (see below). In particular, the day before RS subjects were carefully instructed to collect 24 h urine specimen for CrCl measurement, this latter calculated using SCr and urinary creatinine (Cr) as the following algorithm:
CrCl (in ml/min)=Urine Cr×Urine volume/SCr×minutes.
Fasting serum uric acid and serum lipids were analysed by enzymatic method. Body mass index (BMI) was calculated as the ratio of weight (in kilograms) to squared height (in meters). Waist circumference (in cm) was taken with a tape measure as the point midway between the costal margin and iliac crest in the mid-axillary line, with the subject standing and breathing normally. Body surface area was calculated using the formula of DuBois & DuBois (Citation20).
Blood pressure (diastolic Korotkoff phase 5) was taken in triplicate in lying position using a mercury sphygmomanometer at 10-min intervals, taking special care to avoid any terminal digit preference. The average of the last two clinostatic measurements was taken as BP, to minimize white-coat effects, if any; heart rate was also taken at the same time. Arterial hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or current treatment with antihypertensive drugs (Citation21). Pulse pressure (PP) was the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP). Subjects with fasting glucose between 100 and 125 mg/dl were considered as having impaired fasting glucose (IFG). According to cigarettes smoked, subjects were classified into never and current (≥1 cigarette daily) smokers.
Renal scintigraphy
GFR was measured by Tc99mDTPA clearance (Citation22). The radiopharmaceutical was prepared 30–60 min prior to injection using a current DTPA kit (Astrim, Milano, Italy). The patient was hydrated by 300 ml of water 30 min prior to the examination and was subsequently lay down on a bed in the supine position. Tc99mDTPA was given through an indwelling butterfly needle in an antecubital vein and was followed by infusion of 20 ml of normal saline. The dynamic imaging was acquired in the posterior view by a dual-head automatic body contour gamma camera (Siemens Medical Solution, Hoffman Estates, IL, USA) coupled with a LEAP (low-energy all-purpose) collimator and the energy level set at the Tc-99m 140 keV peak using a 10% window. After bolus intravenous injection of 3 MBq/kg Tc-99m DTPA, dynamic frames in a 128×128 matrix were recorded with an online dedicated work station, initially at 1 s for 1 min and then at 10 s for 20 min. The post-injection syringe with a straight needle was detached before the injection, and was again counted by the two devices in the same way as pre-injection. A region of interest (ROI) was drawn over the renal cortex to exclude the activity in the collecting system. To evaluate perfusion-related and function-related parameters, a renal time–activity curve was generated based on data corrected for extrarenal background activity (Citation23). GFR was measured using counts obtained from the whole kidney ROI cleared by the background ROI, and normalized for BSA.
Formulas for GFR estimation
GFR approximation was estimated (eGFR) using the modified formula of the MDRD as the following algorithm (Citation12):
eGFR (ml/min/1.73 m2) = 186×SCr−1.154×age−0.203×0.742 (if woman).
The eGFR was also calculated from SCr using the CG equation (Citation11):
eGFR (ml/min) = [(140-ageyears) × weightkg)/(SCrmg/dl×72);
the result of this equation was corrected for women by multiplying eGFR by 0.85. GFR values estimated by CG and MDRD formulas was normalized for BSA (in ml/min/1.73 m2).
Statistical analysis
Continuous variables were averaged, expressed as mean and standard deviation (SD), and compared with analysis of covariance and the Bonferroni's post hoc test. Pearson's simple correlation was used to assess relationships between GFR measured by renal scintigraphy (rsGFR) and GFR estimated by ClCr, CG and MDRD formulas (eGFR). Partial correlation analysis was performed to assess the effect of potentially confounding variables on simple correlation. Categorical variables were expressed as percentage rates and compared with the Pearson's χ2 test.
To determine the interchangeably of eGFR against rsGFR Bland–Altman analysis was used (Citation24).
Agreement between methods
The agreement between renal scintigraphy and other estimates of the GFR (derived from ClCr, CG and MDRD formulas) was evaluated by plotting the difference between the two methods on the ordinate (renal scintigraphy minus other methods) and their mean value on the abscissa, according to the Bland–Altman method. If the differences do not vary in any systematic way over the range of measurement in the abscissa and are normally distributed, 95% of differences will lie between the mean difference (the bias)±1.96 SD (the two limits of agreement between the methods); in particular the Bland–Altman method was applied to display the agreement between eGFR and rGFR values by calculating the span between −1.96 and +1.96 SD of the bias. A good agreement between two measurements (meaning interchangeability of measurements) should result in a narrow scatter around zero of the random differences between methods in a direction parallel to the abscissa, representing mean values of both measurements. The Bland– Altman methods was performed as in the whole population rather than separately for reduced (<60 ml/min/1.73 m2) and normal (≥60 ml/min/1.73 m2) rsGFR. The results were considered significant at p≤0.05. Statistical analyses were performed using SPSS package version 13.0 for Windows (SPSS, Chicago, IL, USA).
Results
The general characteristics of study population and the GFR values measured by different methods are summarized in , also showing gender stratification.
Table I. General characteristics of the study population.
Mean age was 62.9±6.6 years, without difference in men than in women (63.1±6.1 and 62.8±7.1 years, NS, respectively). Men compared with women had significantly higher values of SCr, GFR estimated by MDRD formula, waist circumference and history of HT, and a higher prevalence of smoking. Women had higher levels of heart rate, high-density lipoprotein (HDL)-cholesterol as well as higher prevalence of anti-hypertensive treatment.
The univariate correlation showed a significant relationship between rsGFR and eGFR estimated by ClCr, CG and MDRD (). This association remained significant even after adjustment for confounding variables such as age, gender and BMI ().
Figure 1. Pearson's simple correlation of glomerular filtration rate (GFR) measured by technetium-99m-diethyl triamine penta-acetic acid (TCm99DTPA) clearance vs GFR estimated with different methods. (A) Tc99mDTPA clearance vs 24-h creatinine clearance (ClCr); (B) Tc99mDTPA clearance vs Cockcroft–Gault formula; (C) Tc99mDTPA clearance vs Modification of Diet in Renal Disease (MDRD) formula.
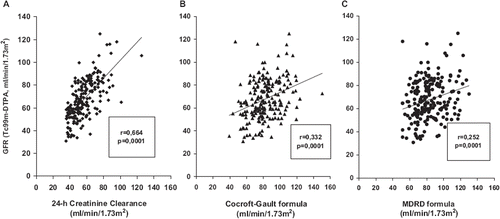
Table II. Univariate and partial correlation analysis of glomerular filtration rate (GFR) measured by renal scintigraphya (rsGFR) with GFR estimated by 24-h creatinine clearance (CrCl), Cockcroft–Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulas.
In all subjects, ClCr significantly overestimated rsGFR (bias +10.8) while this latter was underestimated by CG and MDRD formulas (bias −14.5 and −15.2, respectively). In subjects with normal rsGFR values (, panel A), the Bland–Altman method shown that the smallest span between eGFR and rsGFR was for the ClCr values (26.0 ml/min/1.73 m2), which was somewhat lower than the values of the CG and the MDRD formulas (41. 0 and 40.4 ml/min/1.73 m2, respectively).
Figure 2. Bland–Altman analysis of glomerular filtration rate (GFR) measured by technetium-99m-diethyl triamine penta-acetic acid (TCm99DTPA) clearance renal scintigraphy (rsGFR) and GFR estimates (eGFR) with different methods, in subjects with normal (panel A) and low rsGFR (panel B). The differences between two methods are plotted against the average of Tcm99DTPA clearance and eGFR for each individual patient. (A1, B1) rsGFR vs 24-h creatinine clearance (ClCr); (A2, B2) rsGFR vs Cockcroft–Gault (CG) formula; (A3, B3) rsGFR vs Modification of Diet in Renal Disease (MDRD) formula. The mean difference is indicated by the line, limits of agreement are indicated by the dotted lines.
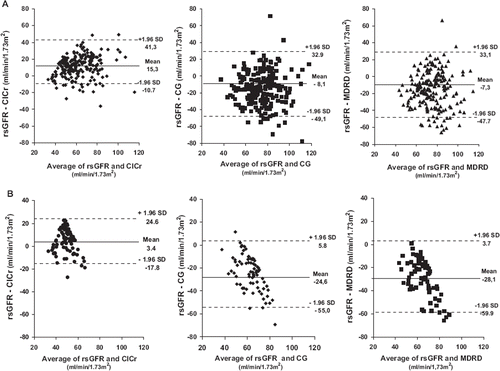
In 76 subjects, an unknown impairment of GFR (<60 ml/min/1.73 m2) was discovered by Tc99mDTPA renal scintigraphy. As observed in subjects with normal rsGFR, and also in subjects with impairment of renal function, ClCr significantly overestimated rsGFR (bias +3.4) while CG and MDRD formulas underestimated rsGFR (bias −24.6 and −28.1, respectively). As shown in (panel B), the smallest span was found for ClCr (21.2 ml/ min/1.73 m2) than CG and MDRD formulas (30.4 and 31.8 ml/min/1.73 m2, respectively). The degree of agreement for eGFR derived from CG and MDRD formulas were wider than GFR estimated by ClCr, reflecting a great variation of the differences and a considerable discrepancy on rsGFR values in the former than in the latter (). As a consequence, CrCl is a good interchangeable method of GFR measured by renal scintigraphy. In both genders, rsGFR was significantly overestimated by CrCl and underestimated by CG and MDRD formulas as in men (+12.9, −14.3 and −18.5 than in women (+8.8, −14.4 and −12.2); the same results was observed for subjects with low renal function (data not shown).
Table III. Bias and interchangeability of methods estimating the glomerular filtration rate (GFR) by 24-h creatinine clearance (ClCr), Cockcroft–Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulas against GFR measured by technetium-99m diethyl triamine penta-acetic acid (Tc99mDTPA) with renal scintigraphy, in the entire population and for GFR categories.
Discussion
The diagnosis of hypertension-induced CKD is based of a reduced renal function and/or the detection of elevated urinary albumin excretion rate (Citation25).
SCr is the most widely used direct measure of reduced renal function and its popularity is attributable to convenience and low cost, but unfortunately, it is very insensitive to evaluate an early decline of renal function (Citation3). SCr is mainly produced by the metabolism of creatine in muscle, but also originates from dietary sources of creatinine such as cooked meat (Citation26). Creatinine generation from the muscles is proportional to the total muscle mass and muscle catabolism, and in people with a relatively low muscle mass, including children, women, elderly, malnourished and cancer patients, the SCr is lower for a given GFR (Citation27). As a consequence, there is a risk of underestimating the amount of renal impairment in these patients, as their SCr is also relatively lower. For example, the GFR may be reduced as low as 20–30 ml/min in a small elderly woman, whereas her SCr remains in the upper range of normal. In addition, the correct interpretation of SCr in the clinical setting is problematic, as frequently the variation in SCr production related to differences in muscle mass leads to misinterpretation of SCr levels. This confusion may be compounded by the use of standard normal ranges for SCr levels that appear on routine laboratory reports (Citation28). As a consequence, the prevalence of CKD is severely underestimated when it is defined according to SCr levels and the most accurate way to assess renal function is to measure the GFR (Citation29). Furthermore, GFR measured by more accurate techniques (described later) may be reduced by up to 50% before SCr becomes elevated (Citation30).
The GFR is the rate at which an ultrafiltrate of plasma is produced by the glomerulus per unit of time and it is the best estimate of functioning nephrons and renal mass (Citation31). GFR is reduced before the onset of symptoms of renal impairment and is related to the severity of the structural abnormalities in CKD. The accurate measurement of GFR in people without overt nephropathy is considered time-consuming and expensive (Citation32) and as a consequence, in clinical practice GFR is commonly evaluated by the measurement of ClCr. However, measuring ClCr obviates some of the problems of using SCr as a marker of GFR but creates others (Citation33). Differences in steady-state creatinine production because of differences in muscle mass that affect SCr should not affect CrCl and extrarenal elimination of creatinine should also have little influence on the ability of ClCr to estimate GFR (Citation34). However, the reliability of CrCl is greatly diminished by variability in tubular secretion of creatinine and by the inability of most patients to collect accurately timed urine samples. As a consequence, some authors have argued that ClCr is a less reliable measure of GFR than SCr and that its use should be abandoned (Citation35,Citation36).
In clinical practice, another option to estimate GFR is to use some formulas that include patient's age, sex, height and SCr. The more known are the abreviated MDRD (Citation12) and CG formulas (Citation11), but their accuracy in clinical practice has been questioned. Both formulas help to detect mild impaired renal function in face of SCr values that are still in the normal range but have the “common bias” to include in their computation SCr value that, as mentioned above, it is a poor predictor of the real GFR.
In our experience, both MDRD and CG formulas underestimated renal function compared than measured by Tc99mDTPA clearance. A possible explanation of this finding is that both MDRD and CG formulas have been tested in patients with high prevalence of CKD and as a consequence, are not applicable in apparently healthy people without known renal disease like in our subjects (Citation37). Furthermore, the MDRD formula has not been validated in subjects younger 18 or older than 70 years (Citation38). Indeed we can speculate that the lack of association between GFR and abbreviate MDRD formula used in this study is in part related to the lower precision of this formula than the original MDRD equation that includes six variables for its computation (Citation39).
In the same manner our results confirm the opinion that the use of the CG formula has significant limitations when an accurate assessment of renal function is required (Citation40). It is common opinion that CG formula is adequate for clinical application, but its significance is limited in subjects with normal renal function (Citation41,Citation42). In our experience, the negative value of mean bias (−14.4, 95% CI 17.2–12.6%) found for the CG formula results in the whole population in a statistically significant underestimation of GFR measured by Tc99mDTPA (Citation43). This indicates that in HTs with normal SCr value, the estimate of GFR provided by the CC formula may underestimate true renal function by as much as 12%, reaching even the 21% in subjects with moderately impairment of renal function (i.e. 59.9 to 30.1 ml/min/1.73 m2).
In spite of MDRD and CG formulas, CrCl overestimated the GFR measured by Tc99mDTPA more in HTs with normal (mean bias +15.3) than in those with low renal function (mean bias +3.4). However, in the latter, the degree of agreement was very much lower than that observed for MDRD and and CG formulas, demonstrating that CrCl is a good interchangeable method for GFR measured by renal scintigraphy. The overestimation of GFR found in our HTs by ClCr measurement is in part related to the increase in tubular secretion of creatinine that was observed with the impairment of renal function (Citation44). However, this overestimation of GFR is limited when both SCr and urine creatinine are both measured by the Jaffe method (Citation19). There are some plasma constituents that tend falsely to raise the SCr measured by the Jaffe assay, whereas urine creatinine levels are largely unaffected (Citation45,Citation46). The low ClCr values found in our HTs with apparently normal SCr are in part to ascribe of the physiological impairment of GFR that declines with age at an annual rate of 1 ml/min/1.73 m2 from the age of 40. However, an incomplete 24-h urine collection may limit the accuracy of ClCr estimation as reducing their values. Furthermore, as GFR varies according to renal mass and correspondingly to BMI, in our study the crude values of CrCl were corrected for BSA thus reducing their levels; it is known that the correction to BSA leads to 12% lower results (Citation47). To resolve this question, it would be useful to evaluate in our HTs the cystatin C level that is considered a more reliable measure of GFR than ClCr (Citation48), but our laboratory does not offer this test routinely.
The generally accepted gold standard for GFR estimation was the inulin clearance, but this method was expensive, time-consuming and not routinely used in clinical practice. Measurement of radioactive-labelled tracer clearance after a single injection has emerged as an alternative to inulin clearance. Among the radio-labelled markers, Tc99mDTPA is relatively inexpensive, is convenient to prepare, provides a low radiation dose to patients (like as a chest radiogram) and can be used for GFR measurement. It has been shown that the multiple plasma samples method for GFR determination following a single injection of Tc99mDTPA was identical to inulin clearance (Citation49,Citation50). Research indicated that the dual blood sampling method significantly correlated with the multiple blood sampling method (R=0.996, standardized estimation error=2.8 ml/min) (Citation51) and was used as reference GFR in clinical trials as recommended by the Nephrology Committee of Society of Nuclear Medicine (Citation52). As a consequence, Tc99mDTPA renal scintigraphy was chosen as reference standard in our study.
In HTs, renal scintigraphy reminds us to test for renovascular hypertension, but when there is not a strong suspicion for it, most physicians forget that renal scintigraphy exists. In our experience, performed in HTs with apparently normal SCr levels, in 38% of cases, GFR measured by Tc99mDTPA renal scintigraphy was able to discover unknown renal organ damage (GFR<60 ml/min/1.73 m2).
In conclusion, GFR measured by Tc99mDTPA renal scintigraphy is mildly and moderately reduced in the main part of non-diabetic hypertensive patients without CV complications and with normal SCr values. This study demonstrates that renal function estimated by CG and MDRD formulas provide a biased and imprecise estimate of GFR. Although the real prevalence of renal function impairment is strongly influenced by methods used to estimate the GFR, in our experience it is surprising high. This observation seems to confirm that renal injury may be detectable in HTs more often than previously thought. When an accurate estimate of GFR is required, clearance of Tc99mDTPA should be measured, but if this methodology is not available, ClCr measurement is the preferred tool to estimate GFR. On the contrary, further research is required to develop more reliable formulas for estimating the true renal function in HTs with normal SCr values. In the mean time, Tc99mDTPA renal scintigraphy should be recommended for the HTs with a mean age of 63 years to detect pre-clinical organ renal damage early, a condition that is more likely to develop in HTs coronary heart disease and CV events than is reported for people of the general population (Citation53). Because its rather low costs (even free for subjects with documented diagnosis of hypertension), easy performance and high accuracy, Tc99mDTPA renal scintigraphy could be used safely, thereby providing clinicians a further tool to better assess the global CV risk of their patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Weiner DE, Krassilnikova M, Tighiouart H, Salem DN, Levey AS, Sarnak MJ. CKD classification based on estimated GFR over three years and subsequent cardiac and mortality outcomes: A cohort study. BMC Nephrol. 2009;17: 10–26.
- Chanda R, Fenves AZ. Hypertension in patients with chronic kidney disease. Curr Hypertens Rep. 2009;11:329–336.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38:1933–1953.
- Culleton BF, Larson MG, Evans JC. Prevalence and correlates of elevated serum creatinine levels: The Framingham heart study. Arch Intern Med. 1999;159:1785–1790.
- Giovannetti S, Barsotti G. In defense of creatinine clearance. Nephron. 1991;59:11–14.
- Luke DR, Halstenson CE, Opsahl JA, Matzke GR. Validity of creatinine clearance estimates in the assessment of renal function. Clin Pharmacol Ther. 1990;48:503–508.
- McDermott DF, Galindo A, Sherman RL . Inadequacy of predicted creatinine clearance as guide to chemotherapy. Cancer Treat Rep. 1987;71:1067–1069.
- Nozue T, Michishita I, Iwaki T, Mizuguchi I, Miura M. Contrast medium volume to estimated glomerular filtration rate ratio as a predictor of contrast-induced nephropathy developing after elective percutaneous coronary intervention. J Cardiol. 2009;54:214–220.
- Jelliffe RW. Creatinine clearance: Bedside estimate. Ann Intern Med. 1973;79:604–605.
- Stevens LA, Levey AS. Impact of reporting estimated glomerular filtration rate: It's not just about us. Kidney Int. 2009; 76:245–247.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Levey AS, Bosch JP, Lewis JB. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Botev R, Mallié JP, Couchoud C, Schück O, Fauvel JP, Wetzels JF, . Estimating glomerular filtration rate: Cockcroft–Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899–906.
- Delanaye P, Cohen EP. Formula-based estimates of the GFR: Equations variable and uncertain. Nephron Clin Pract. 2008; 110:c48–c53.
- Taylor A. Radionuclide renography: A personal approach. Semin Nucl Med. 1999;29:102–127.
- Morton KA, Pisani DE, Whiting JH Jr, Cheung AK, Arias JM, Valdivia S. Determination of glomerular filtration rate using technetium-99m-DTPA with differing degrees of renal function. J Nucl Med Technol. 1997;25:110–114.
- Rehling M, Moller ML, Lund JO. Simultaneous measurement of Tc-99mDTPA, Cr-51 EDTA and inulin in man. Clin Sci. 1984;66:613–619.
- Leoncini G, Viazzi F, Conti N, Baratto E, Tomolillo C, Bezante GP, . Renal and cardiac abnormalities in primary hypertension. J Hypertens. 2009;27:1064–1073.
- Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46:53–55.
- DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, . 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and of the European Society of Cardiology. J Hypertens. 2007;25:1105–1187.
- Buck A C, Macleod MA, Blacklock NJ. The advantages of 99mTc DTPA in dynamic renal scintigraphy and measurement of renal function. Br J Urol. 2008;52:174–186.
- Peters AM, Ussov W Yu. Simultaneous measurement of extracellular fluid distribution and renal function with a single injection of 99mTc DTPA. Nephrol Dial Transplant. 1995; 10: 1829–1833.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – Measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483.
- Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983; 37:478–494.
- Ozmen S, Kaplan MA, Kaya H, Akin A, Danis R, Kizilkan B, . Role of lean body mass for estimation of glomerular filtration rate in patients with chronic kidney disease with various body mass indices. Scand J Urol Nephrol. 2009; 43:171–176.
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, . Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18.
- Rose BD. Clinical assessment of renal function. Rose BD. Pathophysiology of renal disease. 2nd. New York: McGraw-Hill; 1987. 1–23.
- Thomas L, Huber AR. Renal function – Estimation of glomerular filtration rate. Clin Chem Lab Med. 2006;44: 295–1302.
- Hallan S, Astor B, Lydersen S. Estimating glomerular filtration rate in the general population: The second Health Survey of Nord-Trondelag (HUNT II). Nephrol Dial Transplant. 2006;21:1525–1533.
- Rocco MV, Berns JS. KDOQI in the era of global guidelines. Am J Kidney Dis. 2009;54:781–787.
- Demirovic JA, Pai AB, Pai MP. Estimation of creatinine clearance in morbidly obese patients. Am J Health Syst Pharm. 2009;66:642–648.
- Sawyer WT, Canaday BR, Poe TE, Webb CE, Gal P, Joyner PU, Berry JI. Variables affecting creatinine clearance prediction. Am J Hosp Pharm. 1983;40:2175–2180.
- Soares AA, Eyff TF, Campani RB, Ritter L, Camargo JL, Silveiro SP. Glomerular filtration rate measurement and prediction equations. Clin Chem Lab Med. 2009;47:1023–1032.
- Miller WG. Estimating glomerular filtration rate. Clin Chem Lab Med. 2009;47:1017–1019.
- Itoh K. Comparison of methods for determination of glomerular filtration rate: Tc-99m-DTPA renography, predicted creatinine clearance method and plasma sample method. Ann Nucl Med. 2003;17:561–565.
- Delanghe JR. GFR. Were are we now? JIFCC. 2009;20:66–71.
- Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, . Evaluation of the Modification of Diet in Renal Disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757.
- Martin L, Chatelut E, Boneu A. Improvement of the Cockcroft–Gault equation for predicting glomerular filtration in cancer patients. Bull Cancer. 1998;85:631–636.
- Toto RD. Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens. 1995;4:505–509.
- Cochran S, St John A. A comparison between estimates of GFR using [99mTc]DTPA clearance and approximation of Cockcroft and Gault. Aust NZ J Med. 1993;23:494–497.
- Levey AS, Bosch JP, Breyer Lewis J. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130: 461–470.
- Argani H, Dabiri S, Taghizadeh M, Pishahang P, Rezvan NH, Emami P. Estimation of glomerular filtration rate in renal transplants based on serum creatinine level after oral cimetidine. Transplant Proc. 2000;32:545–546.
- Shemesh O, Golbetz H, Kriss JP. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838.
- Biörk J, Bäck SE, Sterner G, Carlson J, Lindström V, Bakoush O, . Prediction of relative glomerular filtration rate in adults: New improved equations based on Swedish Caucasians and standardized plasma creatinine assays. Scand J Clin & Lab Invest. 2007;67:678–695.
- McIntyre CW, Selby NJ. Indexing glomerular filtration rate for body surface area is useful in obese subjects. Nephrol Dial Transplant. 2006;21:821.
- Kyshe-Anderson J, Schmidt C, Nordin G Serum Cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum glomerular filtration rate. Clin Chem. 1994;40:1921.
- Rehling M, Moller ML, Thamdrup B, Lund JO, Trap-Jensen J. Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci. 1984;66:613–619.
- Cousins C, Gunasekera RD, Mubashar M, Mohammadtaghi S, Strong R, Myers MJ. Comparative kinetics of microvascular inulin and 99mTc-labelled diethylenetriaminepenta -acetic acid exchange. Clin Sci. 1997;93:471–477.
- Waller DG, Keast CM, Fleming JS. Measurement of glomerular filtration rate with technetium-99m DTPA: Comparison of plasma clearance techniques. J Nucl Med. 1987;28:372–377.
- Blauox MD, Aurell M, Bubeck B. Report of the radionuclides in nephrourology committee on renal clearance. J Nucl Med. 1996;37:1883–1890.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351: 1296–1305.