Abstract
Objective. Enhanced external counterpulsation (EECP) is a non-invasive technique that has been shown to reduce the frequency and severity of angina pectoris. Little is known how EECP affects the blood pressure. Methods. 153 patients with refractory angina were treated with either EECP or retained on their pharmacological treatment (reference group). Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP) and heart rate were measured pre- and post-treatment and at 12 months follow-up. Results. EECP treatment altered the blood pressure in patients with refractory angina pectoris. A decrease in the blood pressure was more common in the EECP group compared with the reference group. In the reference group, an increase in the blood pressure was more common. A correlation between a decrease in blood pressure after EECP treatment and a higher baseline MAP, SBP and DBP was seen. No such correlation was seen in the reference group. The blood pressure response did not persist at 12 months follow-up. Conclusion. EECP treatment affects the blood pressure in patients with refractory angina pectoris. The decreased blood pressure may be a result of an improved exercise capacity, an improved endothelial function and vasoreactivity in general.
Introduction
Patients with coronary artery disease (CAD) often have multiple medical therapies including several invasive interventions. Despite current therapy, some patients have highly symptomatic CAD and suffer from severe angina pectoris, which cannot be controlled by a combination of medical therapy and revascularization. Refractory angina pectoris is characterized by chronic angina related to coronary artery insufficiency in patients who are refractory to conventional treatments (Citation1). Enhanced external counterpulsation (EECP) is a non-invasive technique that has been shown to reduce angina pectoris in CAD. The therapy utilizes basic hemodynamic principles to treat refractory angina pectoris (Citation2), although its effect on blood pressure is not well known. The results from previous studies show a positive clinical response among treated patients (Citation2–5).
The basic principle of EECP is diastolic augmentation of arterial pressure, lowering of systolic arterial pressure along with increasing venous return. The systolic blood pressure (SBP) is lowered because of deflation before systole, thereby decreasing afterload (Citation6). Still, the physiological mechanism of benefit with EECP remains unclear. Proposed mechanisms behind the clinical benefit include promotion of coronary artery collateral flow, a training-like effect and improved endothelial function (Citation7,Citation8). Heart rate variability has been studied as a non-invasive marker of autonomic tone (Citation9). However, improved heart rate has not been found to correlate to anti angina benefits of EECP (Citation10).
Patients with CAD may have several cardiovascular risk factors such as diabetes mellitus and hypertension. These conditions characterize deteriorated quality of the large arteries (Citation11,Citation12). These differences may have pathophysiological relevance, since some patients do respond to EECP treatment; others do not (Citation13). The pathophysiological explanation to the clinical improvements over time, experienced by patients suffering from refractory angina pectoris treated with EECP, is still not fully understood. Although blood pressure is of importance in patients with CAD, little is known about the systemic blood pressure response following EECP treatment. Based on the positive hemodynamic effects of EECP with reduced afterload, increased shear stress and venous return, it is frequently asked if altered blood pressure may be of pathophysiological relevance, since arterial wall qualities play a role in arterial impedance, left ventricular afterload and myocardial oxygen consumption.
Our hypothesis is that EECP treatment positively alters or correlates with the blood pressure response in patients with refractory angina pectoris compared with a group of patients with refractory angina pectoris only receiving pharmacological treatment.
Aim
The present study aims to evaluate the arterial blood pressure response immediate and at 12 months follow-up in patients with refractory angina pectoris treated with EECP and to compare the results to a group of refractory angina patients only receiving the same pharmacological treatment.
Materials and methods
Patients
A total of 153 patients with refractory angina pectoris were included in the study; 100 consecutive patients (77 males and 23 females), 47–91 years old, were referred to EECP treatment because of refractory angina pectoris, agreed to participate and were included in the study. The remaining, 53 patients, with similar symptoms (41 males and 12 females), 52–87 years old, did not receive EECP because of contraindications (deep vein thrombosis, fast irregular rhythms, severe hypertension, peripheral vascular disease or severe aortic insufficiency) or unwillingness to undergo EECP treatment. These patients were retained on their pharmacological treatment and included as a reference group (). Treatment was carried out at the department of Medicine at the Central Hospital in Kristianstad (Sweden). All patients had angiographically documented CAD with verified stenoses in at least one major coronary artery. The patients were considered at optimal pharmacological treatment and not suitable for further revascularization. Optimal medical therapy includes the maximum tolerated use of anti angina medications (long and short-acting nitrates, β-adrenoceptor antagonists and/or Ca2+ antagonists). Entering the study, data on demographics, medical history and current medication were collected from the patients (). All medications remained unchanged during the entire study period but could, if needed, be adjusted. All patients suitable for this study approved their inclusion and signed an informed consent. The study was conducted in accordance with the declaration of Helsinki. The study was approved by the ethical committee of Lund University (D nr 410/ 2007).
Table I. Baseline characteristics.
Enhanced external counterpulsation – technique
EECP (Vasomedical Inc. Täby, Sweden) is a therapeutic system that consists of an air compressor, a treatment table, a control console and an integrated set of air cuffs. These compressive cuffs are designed to be wrapped around the patient's lower extremities. The EECP therapist, a specialized nurse sets cuff inflation and deflation to the cardiac cycle guided by the electrocardiogram (ECG); systolic and diastolic pressure augmentation during EECP is monitored using finger plethysmography (Citation14). Cuffs are timed to inflate sequentially from the calf to the lower thigh to the upper thigh and buttock just after the onset of diastole, and then deflate simultaneously prior to the beginning of systole. Pressures applied to the cuffs range from 80 to 300 mmHg. Pressure applied in this study was 260 mmHg. A typical course of EECP therapy consists of 35 h of treatment, 1 h per day, 5 days per week, over a 7-week period.
Pharmacological therapy
The standard treatment for symptomatic relief in patients with chronic stable angina pectoris includes a long-acting nitrate, β-adrenoceptor antagonists and/or Ca2+ antagonists titrated to the lowest heart rate and blood pressure level tolerated ().
Clinical parameters in treatment
EECP treatment was carried out within 12 weeks from the inclusion of the patients. All patients underwent 35±2 h of EECP, each session lasting 1 h. The treatment period lasted 35 days. SBP, diastolic blood pressure (DBP) and heart rate were registered before the first EECP session (baseline) and after the last EECP session, and at 12 months after the final EECP session. Resting brachial blood pressure measurements were obtained by EECP technicians and made by digital palpation of the brachial artery using an automated recorder (ARGUS VCM, Baar, Switzerland). Recorded ECG followed the heart rate. The blood pressure response was measured after 7 weeks of EECP treatment and at 12 months after the final session. Assessment of treatment effect was blood pressure as measured by SBP, DBP and mean arterial blood pressure (MAP). Patients in the reference group were registered similar with SBP, DBP and heart rate at baseline, after 7 weeks and at 12 months follow-up. In this study, we estimated 5 mmHg as a significant clinical change in blood pressure (Citation15). When at least two of three (SBP, DBP or MAP) blood pressures were changed in a patient at the different follow-ups, the patient was defined as a patient with an overall change in blood pressure. Thus, when no more than one blood pressure (SBP, DBP or MAP) was changed at the different follow-ups, the patient's overall blood pressure was defined as unchanged. Recorded ECG followed the heart rate. Other data like medication were collected from the medical records and clinic visits at baseline with follow-up post-EECP or 7 weeks for the controls and 12 months after respective therapy. During the study period, angina status was estimated using Canadian Cardiovascular Society (CCS) angina scale I–IV for angina pectoris (Citation16). One cardiologist and one specialized nurse performed CCS-classification separately; their combined judgement was used in the study. Adverse events were recorded as soon as they were reported.
Statistical analysis
All calculations and statistics were performed by using the software program GraphPad Prism 4.0. Values are presented as means±standard deviation. Statistical comparisons between groups were performed by the unpaired Student t-test when comparing two groups. Analysis of variance (ANOVA) with Dunnett's post hoc correlation was used when comparing more than two groups. Correlations were analyzed with Fishers exact test. Statistical significance was accepted when p<0.05.
Results
The baseline demographics and clinical characteristics are presented in . The baseline characteristics were similar in patients treated with EECP and patients only receiving pharmacological treatment. There were few differences; somewhat more patients undergoing EECP were treated with β-adrenoceptor antagonists and had a lower SBP at baseline compared with the patients that were only treated pharmacologically. The anti-angina medications were unchanged during the 12 months follow-up.
The EECP treatment was well tolerated. No complications occurred and all patients included in the study completed their treatment. However, because of logistics three patients completed after 33 h of treatment and two patients completed after 34 h of treatment.
Immediately after EECP treatment, the overall blood pressure was altered in the majority of patients with refractory angina pectoris compared with patients only receiving pharmacological treatment (92% vs79%, p<0.05; ). More patients changed their MAP (82 vs 68%) and their SBP (83 vs 68%) in the EECP group compared with the reference group (p<0.05; ). The change constituted mainly a drop in the pressure. There was no difference in the percentage of patients that changed their DBP between the EECP treated patients and the patients only receiving pharmacological treatment (p=n.s.).
Figure 1. Diagram showing the overall blood pressure change of participants through the study comparing enhanced external counterpulsation (EECP) and controls. When at least two of three [systolic (SBP), diastolic (DBP) or mean arterial (MAP)] blood pressures were changed in a patient at the different follow-ups, the patient was defined as a patient with an overall change in blood pressure. Thus, when only one blood pressure (SBP, DBP or MAP) was changed at the different follow-ups, the patient's overall blood pressure was defined as unchanged. All values are presented as mean±SEM. *p<0.05.
![Figure 1. Diagram showing the overall blood pressure change of participants through the study comparing enhanced external counterpulsation (EECP) and controls. When at least two of three [systolic (SBP), diastolic (DBP) or mean arterial (MAP)] blood pressures were changed in a patient at the different follow-ups, the patient was defined as a patient with an overall change in blood pressure. Thus, when only one blood pressure (SBP, DBP or MAP) was changed at the different follow-ups, the patient's overall blood pressure was defined as unchanged. All values are presented as mean±SEM. *p<0.05.](/cms/asset/a474c631-8d9a-49d0-a771-06248a1a0746/iblo_a_479959_f0001_b.jpg)
Table II. Percentage of patients in which the blood pressure were changed after enhanced external counterpulsation (EECP) treatment, respectively after solely pharmacological treatment in patients with refractory angina pectoris.
At the 12 months follow-up, there was a tendency that more patients in the EECP group had a changed overall blood pressure from baseline compared with the reference group, although this was not significant (p=0.05; ). Twelve months after treatment, more patients in the EECP group had a different SBP from baseline compared with patients only receiving pharmacological treatment (p<0.05; ). There were a similar number of patients with changed MAP and DBP from baseline in the EECP and in the reference group at the 12 months follow-up (p=n.s.; ).
Patients treated with EECP in which the overall blood pressure was changed after treatment had a significantly higher baseline MAP, SBP and DBP compared with patients who did not change blood pressure (). There was no such correlation in the reference group; MAP, SBP and DBP were similar in patients with changed as well as unchanged blood pressure at the 7-week follow-up (p=n.s.; ). There was no correlation between baseline blood pressure and altered blood pressure response in patients treated with EECP or in patients only receiving pharmacological treatment at the 12 months follow-up (p=n.s.).
Table III. Mean blood pressure (mmHg) in patients treated with enhanced external counterpulsation (EECP) and in patients only receiving pharmacological treatment with changed or unchanged blood pressure after treatment.
Overall, the alterations in blood pressure seen after EECP treatment were unaffected by previous diagnosis, medications, treatments as well as degree of angina. The DBP was altered to a greater extent in male patients (p<0.01) and in patients with a history of revascularization treated with EECP (p<0.05). There was no correlation between background variables and outcome in patients that were only receiving pharmacological treatment.
The change in blood pressure in the EECP and in the reference groups could be both decreased and increased. Thus, a decrease in blood pressure was more common in patients treated with EECP compared with patients on solely pharmacological treatment (66±5% vs 45±8%, p<0.05). The opposite was seen in the reference group, in which the blood pressure was increased in more patients compared with patients treated with EECP (55±8% vs 34±5%, p<0.05).
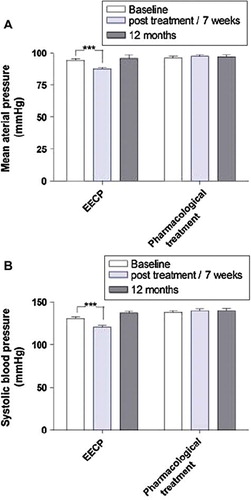
MAP, SBP and DBP were all significantly decreased after EECP treatment (MAP: 94±12 vs 88±11 mmHg; SBP: 131±21 vs 121±16 mmHg; DBP: 74±10 vs 69±10 mmHg, all p<0.001; ). The changes in blood pressure were not persistent at the 12 months follow-up (p=n.s.). There were no changes in blood pressure in the reference group neither at the 7-week follow-up nor at 12 months follow-up (p=n.s.).
Figure 2. Diagram showing the blood pressure response of participants through the study comparing enhanced external counterpulsation (EECP) and controls: (A) mean arterial blood pressure (MAP); (B) systolic blood pressure (SBP) and (C) diastolic blood pressure (DBP). All values were compared with baseline in each group and presented as mean±SEM. ***p<0.001.
Patients that responded with a decrease in blood pressure had higher MAP, SBP or DBP at baseline compared with patients that responded with an increase in blood pressure. This was seen in both the EECP and in the reference groups. On the contrary, patients showing an increase in blood pressure had a significantly lower blood pressure at baseline. Furthermore, the amount of decrease in SBP or MAP (in mmHg) was more pronounced than the increase (SBP 22±13 vs 13±10 mmHg, p<0.05; p<0.05 and MAP 14±8 vs 8±5 mmHg, p<0.01; ). No such differences were seen in DBP (13±6 vs 11±4 mmHg; ). The heart rate was unchanged in patients treated with EECP and in patients only receiving pharmacological treatment at 7 weeks of follow-up (from 67±12 to 65±12 beats/min, p=n.s.).
Table IV. Mean blood pressure (mmHg) in patients treated with enhanced external counterpulsation (EECP) and in patients only receiving pharmacological treatment with decreased or increased blood pressure after treatment.
The overall blood pressure responses were unaffected by the different baseline characteristics shown in . SBP was decreased in more patients with hypertension in the EECP group compared with the reference group (90% versus 65%, p<0.05). Furthermore, SBP and DBP were more often increased in patients in the reference group treated with RAAS-blockade and/or β-adrenoceptor antagonists at 7 weeks of follow-up (p<0.05).
Discussion
This is, to our knowledge, the first study that has compared the effect of EECP treatment on blood pressure and heart rate with solely pharmacological treatment in patients with refractory angina pectoris. The results show that EECP treatment affects the blood pressure in patients with refractory angina. In a positive way; EECP treatment decreases MAP, SBP and DBP compared with patients only receiving optimal tolerable anti-angina pharmacological treatment in which an increase in blood pressure was more common during the period of study. Also, the results show a correlation between the change in overall blood pressure and the blood pressure pretreatment. Patients that responded with a decrease in blood pressure after EECP treatment had a significantly higher blood pressure pretreatment compared with patients in which the blood pressure was increased after EECP. The present study also shows that the blood pressure response did not correlate with the reduction in angina frequency. The patients with lowered, unaltered or elevated response to EECP still had reduction in angina pectoris frequency. Thus, it seems as though the response to EECP is independent of a change in blood pressure.
In a previous study, EECP treatment was shown to decrease SBP in patients with refractory angina pectoris (Citation17). These results are confirmed in our study, however, here we add the dimension on MAP and the analysis of background factors associated with the blood pressure response. Furthermore, we have compared the findings in the EECP group to another group of patients with chronic stable angina pectoris retaining on their pharmacological treatment (a matched control group), which is of considerable importance.
EECP has been found to be an effective treatment for patients with refractory angina pectoris (Citation5), although the mechanism is complex and not fully understood. The hemodynamics of EECP mimic that of an intra-aortic balloon counterpulsation by augmenting diastolic blood flow in multiple vascular beds, including the coronary arteries, and by reducing afterload (Citation18). The exact mechanism responsible for our findings on the blood pressure after EECP therapy is still unknown. Increased coronary blood flow with diastolic augmentation has been demonstrated with transesophogeal echocardiography(Citation19). Furthermore, results suggest that EECP develops and recruits collateral coronary vessels (Citation20). One study in a “closed-chest infarction model” in dogs has revealed increased distribution of microvessels in the infarcted region of the heart by long-term EECP, supporting the hypothesis that EECP promotes collateral vessel development (Citation21). In addition, shear stress and neurohormonal changes may play a role in the clinical improvements experienced by patients treated with EECP (Citation4,Citation22). Shear stress is the product of flow and wall pressure in any vessel and influenced by enhanced blood flow across the endothelial cell lining of blood vessels (Citation23), which may be the compression of the lower extremities during EECP (Citation24). The increase in coronary blood flow by EECP may enhance endothelial shear stress (Citation25), which improves endothelial function by stimulating the release of the vasodilatory mediator nitric oxide and reduces the release of the vasocontractile endothelin-1 (Citation4,Citation20,Citation26). The improved perfusion by EECP might in addition be related to a reported increase of angiogenesis factors, such as vascular endothelial growth factors, basic fibroblast growth factor and hepatocyte growth factor (Citation27). Taken together, our results with blood pressure measurements may represent a physiological response that integrates the mechanisms mentioned, reflecting both improved endothelial function and vasoreactivity.
There was no significant change in heart rate during the study and at follow-up. Therefore, a frank decrease in sympathetic tone by lying supine during EECP treatment does not explain the findings. EECP has been proven to exert peripheral effects similar to physical exercise (Citation7). Thus, an improved exercise capacity may explain the altered blood pressure in this study. The precise mechanisms connecting effects in blood pressure with improvements in exercise tolerance because of EECP treatment has not been clarified. During exercise, there is an immediate vasodilatation of arteries and capillaries in active skeletal muscle tissue because of increased metabolic demands in contrast to tissue that is not involved where peripheral vascular resistance increases. The total result is a decrease in overall systemic vascular resistance. According to Fagard (Citation28), training from three to five times per week during 30–60 min per session at an intensity of about 40–50% of net maximal exercise, performance appears to be effective with regard to blood pressure reduction.
Since the present study showed no correlation between the reduction in angina frequency and changes in blood pressure, the mechanisms of EECP in reducing angina still needs further studies. Thus, the mechanisms thought to be responsible for the improvement of the angina status include promotion of collateral circulation, enhancement of the endothelial function and improved ventricular function (Citation7).
In the present study, the results show that EECP do affect the blood pressure in patients with refractory angina pectoris. More EECP patients decrease MAP, SBP and DPB compared with patients only receiving optimal anti-angina pharmacological treatment in which an increase in blood pressure was more common. Patients that responded with a decrease in blood pressure after EECP treatment had a higher baseline blood pressure compared with patients in which the blood pressure was increased after EECP. At the 12 months follow-up there was a tendency towards that more patients in the EECP group had a changed overall blood pressure from baseline compared with controls, although this was non-significant. The altered effect on blood pressure may reflect both improved endothelial function and vasoreactivity. This may be of interest when guiding clinicians to choose between treatments for patients with refractory angina pectoris. This renders possible options for other patient groups, which are considered for treatment like patients with heart failure. The improved blood pressure changes after EECP may related to reliable underlying mechanisms of action correspond to improved outcomes. Despite improved quality of life and functional capacity EECP may be used to treat patients with heart failure without risking symptoms of hypotension or hypertension.
However, the usefulness of EECP therapy by physicians must be individualized based on their assessment of the totality of EECP therapy data.
Limitations to the study
Blood pressure measurements were available for only 83 (54%) of 153 studied patients (39 EECP patients and 44 controls) in the 12 months follow-up. Though no differences were seen in the available subjects, it cannot be excluded that our findings may be skewed. Although these are the first available data on blood pressure changes related to EECP with a comparable group of patients only receiving pharmacological treatment with follow-up at 12 months. A long-term compared blood pressure response of patients treated with EECP versus pharmacological treatment, however, needs to be further evaluated.
The comparison of EECP and the control in two groups showed that EECP treatment appeared to affect the blood pressure in patients with refractory angina pectoris. However, direct comparison in a double-blinded, randomized manner is required to verify this suggestion.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, . The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23:355–370.
- Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, . The multicenter study of enhanced external counterpulsation (MUST-EECP): Effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol.1999;33:1833–1840.
- Lawson WE, Hui JC, Soroff HS, Zheng ZS, Kayden DS, Sasvary D, . Efficacy of enhanced external counterpulsation in the treatment of angina pectoris. Am J Cardiol. 1992; 70:859–862.
- Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001; 37:93–99.
- Pettersson T, Bondesson S, Cojocaru D, Ohlsson O, Wackenfors A, Edvinsson L. One year follow-up of patients with refractory angina pectoris treated with enhanced external counterpulsation. BMC Cardiovasc Disord. 2006;6:28.
- Suresh K, Simandl S, Lawson WE, Hui JC, Lillis O, Burger L, Guo T, . Maximizing the hemodynamic benefit of enhanced external counterpulsation. Clin Cardiol. 1998;21:649–653.
- Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, . Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41: 1761–1768.
- Bonetti PO, Holmes DR, Jr., Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: What's behind the curtain? J Am Coll Cardiol. 2003;41: 1918–1925.
- Tsuji H, Venditti FJ, Jr., Manders ES, Evans JC, Larson MG, Feldman CL, . Determinants of heart rate variability. J Am Coll Cardiol. 1996;28:1539–1546.
- Michaels AD, Bart BA, Pinto T, Lafferty J, Fung G, Kennard ED. The effects of enhanced external counterpulsation on time- and frequency-domain measures of heart rate variability. J Electrocardiol. 2007;40:515–521.
- Megnien JL, Simon A, Valensi P, Flaud P, Merli I, Levenson J. Comparative effects of diabetes mellitus and hypertension on physical properties of human large arteries. J Am Coll Cardiol. 1992;20:1562–1568.
- Levenson J, Simon AC, Cambien FA, Beretti C. Cigarette smoking and hypertension. Factors independently associated with blood hyperviscosity and arterial rigidity. Arteriosclerosis. 1987;7:572–577.
- Lawson WE, Hui JC, Kennard ED, Barsness G, Kelsey SF. Predictors of benefit in angina patients one year after completing enhanced external counterpulsation: Initial responders to treatment versus nonresponders. Cardiology. 2005;103: 201–206.
- Soran O. A new treatment modality in heart failure enhanced external counterpulsation (EECP). Cardiol Rev. 2004;12: 15–20.
- Tanaka Y, Tada H, Yamashita E, Sato C, Irie T, Hori Y, . Change in blood pressure just after initiation of cardiac resynchronization therapy predicts long-term clinical outcome in patients with advanced heart failure. Circ J. 2009;73: 288–294.
- Campeau L. Letter: Grading of angina pectoris. Circulation. 1976;54:522–523.
- Campbell AR, Satran D, Zenovich AG, Campbell KM, Espel JC, Arndt TL, . Enhanced external counterpulsation improves systolic blood pressure in patients with refractory angina. Am Heart J. 2008;156:1217–1222.
- Michaels AD, Accad M, Ports TA, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106:1237–1242.
- Werner D, Schneider M, Weise M, Nonnast-Daniel B, Daniel WG. Pneumatic external counterpulsation: A new noninvasive method to improve organ perfusion. Am J Cardiol. 1999; 84:950–962, A7–8.
- Masuda D, Nohara R, Hirai T, Kataoka K, Chen LG, Hosokawa R, . Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by (13)N-ammonia positron emission tomography. Eur Heart J. 2001;22:1451–1458.
- Wu G, Du Z, Hu C, Zheng Z, Zhan C, Ma H, . Angiogenic effects of long-term enhanced external counterpulsation in a dog model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2005.
- Soran O, Crawford LE, Schneider VM, Feldman AM. Enhanced external counterpulsation in the management of patients with cardiovascular disease. Clin Cardiol. 1999;22: 173–178.
- Molineux G KO, Briddell B, Hartley C, McElroy P, Kerzic P, Sutherland W, . A new form of filgrastim with sustained duration in vivo and enhanced ability to mobilize pbpc in both mice and humans. Exp Hematol. 1999;27:1724–1734.
- Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, . Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation. 2007;116:526–534.
- Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, . A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol. 1999;27:1724–1734.
- Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol. 1993;264:H150–156.
- Kersten JR, Pagel PS, Chilian WM, Warltier DC. Multifactorial basis for coronary collateralization: A complex adaptive response to ischemia. Cardiovasc Res. 1999;43: 44–57.
- Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33:S484–492; discussion S93–94.