Abstract
Ten years ago Forkhead box P3 (FOXP3) was discovered as master gene driving CD4+CD25+ T cell regulatory (Treg) function. Since then, several layers of complexity have emerged in the regulation of its expression and function, which is not only exerted in Treg cells. While the mechanisms leading to the highly selective expression of FOXP3 in thymus-derived Treg cells still remain to be elucidated, we review here the current knowledge on the role of FOXP3 in the development of Treg cells and the direct and indirect consequences of FOXP3 mutations on multiple arms of the immune response. Finally, we summarize the newly acquired knowledge on the epigenetic regulation of FOXP3, still largely undefined in human cells.
INTRODUCTION
Primary immunodeficiency disorders (PIDs) are rare genetic diseases of the immune system primarily characterized by recurrent infections and often associated with malignancies and autoimmune manifestations [Citation1]. This dichotomy of overreaction to self-antigens, while lacking a robust response against pathogens suggests that PID patients have a dysregulated immune system, resulting in a decreased ability to distinguish between self and foreign antigens. Autoimmune manifestations associated with reduced number and/or function of regulatory T (Treg) cells, key players in maintaining peripheral tolerance, have been demonstrated in several autoimmune diseases, such as Type 1 Diabetes (T1D) [Citation2], and Systemic Lupus Erythematosus (SLE) [Citation3]. In addition, dysfunction of Treg cells has been detected in several PIDs, such as Adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID) and Wiskott–Aldrich syndrome (WAS), and suggested as the cause of the autoimmune manifestations, further supporting the association of immunodeficiencies and immunedysregulation [Citation4, 5].
The best example of PID with prevailing autoimmune manifestations is the Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) syndrome, due to the loss of function of thymus-derived (t) Treg cells [Citation6], the key cell subset that controls immune responses to pathogens and prevents immune responses against inappropriate targets, such as self-antigens or non-harmful antigens [Citation7]. The disease is caused by mutations in the FOXP3 gene [Citation8], encoding for a T-cell specific transcription factor (TF) belonging to the large forkhead family of TFs. FOXP3 is considered a key player for the specification and function of tTreg cells [Citation7]. Their dysfunction leads to life-threatening autoimmune manifestations in several organs in affected male children [Citation6, Citation9].
In this review, we summarize the current knowledge on the function of FOXP3 in the regulation of Treg and T effector (Teff) cells, with special attention to the consequences of its dysfunction.
FOXP3 EXPRESSION IN AND OUTSIDE THE IMMUNE SYSTEM
Role of FOXP3 in Treg Cells
In the initial studies performed in mice, Foxp3 was reported to be exclusively expressed by CD4+CD25+ Treg cells and its expression was associated with the acquisition of suppressive properties typical of this T-cell subset [Citation10]. Later studies showed that ectopic Foxp3 expression in Teff cells induced the majority of tTreg cell signature genes [Citation11]. On the other hand, Foxp3 expression per se is not sufficient to activate the complete Treg transcriptional program [Citation11, 12] and analysis of foxp3gfpko mice demonstrated that most of the tTreg signature genes are maintained in the absence of Foxp3 expression [Citation13]. These recent findings pointed out that Foxp3 expression is essential, but per se insufficient for the development of bona fide tTreg cells and paved the way for the identification of additional genes contributing to Treg lineage specification.
Studies in humans confirmed that FOXP3 is expressed by tTreg cells and crucially controls their function and maintenance [Citation7], as also demonstrated by the observation that high and stable expression of FOXP3 in human Teff cells leads to the acquisition of Treg phenotype and functions [Citation14, 15].
FOXP3 Expression and Treg Cell Plasticity
There is general consensus on the concept that high and constant expression of FOXP3 is required for the stability of Treg cells [Citation16]. Indeed, several evidences demonstrate that loss of FOXP3 leads to diminished suppressive capacity in both human and murine Treg cells [Citation13, Citation17–20]. In addition to the loss of suppressive function recent studies suggested that loss of FOXP3 expression in Treg cells could switch them from a regulatory fate to the acquisition of pro-inflammatory properties. In murine models it has been proposed that Treg cells can acquire effector functions and become auto-reactive, although the latter phenomenon is still controversial [Citation21]. In an in vivo study, thanks to the use of fate-mapping systems, Zhou and colleagues showed that murine “ex-Treg cells” that have lost Foxp3 expression switched to a Teff phenotype, which contribute to the onset of autoimmunity as a consequence of Foxp3 instability [Citation22]. Loss of FOXP3 with acquisition of inflammatory cytokine production after repeated in vitro stimulation can also be observed in human Treg cells; several studies indicated that specific subsets of human Treg cells could lose regulatory activity and become effector memory T cells producing IL-17, under specific culture conditions [Citation23–26], or could convert to a prevalent Th2 phenotype [Citation27]. This new concept of Treg plasticity has been proposed as a physiological mechanism to allow efficient response to pathogens or to restrain detrimental immune regulation, but it also suggests a potential dual role, both regulatory and pro-inflammatory, for these cells in a variety of disorders, including autoimmunity, cancer, and infections (reviewed in [Citation21]).
However, other in vivo works suggested that tTreg cells are functionally and phenotypically stable after many cell divisions in inflammatory sites [Citation28, 29], and differences in the in vivo outcome (Treg stability vs generation of ex-Treg cells) has been attributed to limitations of the fate mapping systems or of the experimental conditions [Citation30].
More recently, the attention has been shifted away from this controversy by the emerging concept that tTreg lineage commitment is regulated by a complex network of cooperative and counteractive TFs [Citation31]. Treg function can be fine tuned through adoption of inflammatory T helper transcription factor networks, which lead to the generation of Th-like Tregs in both mice and humans [Citation32]. This issue is further discussed in the review by Vent-Schmidt et al.
Role of FOXP3 in T Effector Cells
In murine T cells Foxp3 expression was originally described as restricted to CD4+CD25+ Treg cells [Citation10]. More recently, Miyao et al. reported unstable expression of Foxp3 in activated murine Teff cells both in vitro and in vivo, regardless of the acquisition of classical Treg-related functions [Citation29]. The physiological meaning of heterogeneous Foxp3 expression in murine conventional T cells has not yet been clarified. On the other hand, it has been known for a long time that in humans FOXP3 expression is not restricted to CD4+CD25+ Treg cells, but it can also be transiently induced in activated CD4+CD25− Teff cells, which do not express FOXP3 in the resting state [Citation33, 34]. While the role of FOXP3 as key regulator of human Treg cells has been widely accepted, its function in CD4+CD25− Teff cells remains poorly defined. Also in humans, activation-induced expression of FOXP3 in CD4+CD25− Teff cells is transient and does not necessarily result in the acquisition of suppressive properties [Citation33, 34], although it has been associated with suppression of the cytokine production and proliferation of multiple CD4+ T cell lineages [Citation33, Citation35]. Indeed, FOXP3-deficient Teff cells proliferate more, produce more cytokines than wild type Teff cells, and activation-induced FOXP3 expression is sustained in Th17 cells, suggesting that FOXP3 contributes to the developmental program of human Th17 cells [Citation35].
Role of FOXP3 in Somatic Cells
Although FOXP3 protein expression was originally reported to be restricted to haematological tissues and in particular to CD3+ T lymphocytes, it has been recently described that FOXP3 expression can occur also in non-immune cells, such as epithelial cells, where it functions as a tumor suppressor and is involved in metastatic spread [Citation36, 37]. The original observation that female mice heterozygous for the mutation of the foxp3 gene (foxp3sf/+) developed cancer (i.e. mammary carcinoma) at higher frequency than wild type mice, led to the recognition of FOXP3 as an X-linked breast cancer suppressor, via repression of the oncogenes ERBB2/HER2 and SKP2 [Citation37, 38] both in mice and humans. Subsequent studies demonstrated FOXP3 expression is not specific for breast cancer, where it correlates with metastasis formation [Citation36], but is also expressed by a wide variety of tumor cells, including melanoma [Citation39] and prostate cancer cells [Citation40], confirming the relevant function of FOXP3 as tumor suppressor gene. However, despite increasing evidence for a role in suppressing transformation of epithelial cells, the physiological functions of FOXP3 outside the Treg cell compartment are still poorly defined.
IMMUNE DYSREGULATION, POLYENDOCRINOPATHY, ENTEROPATHY, X-LINKED (IPEX) SYNDROME
Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) is a rare autoimmune disease characterized by early-onset systemic autoimmunity. The distinctive features of the disease comprise: (i) life-threatening enteropathy with refractory diarrhea, usually associated with villous atrophy, (ii) early-onset T1D, and (iii) dermatitis frequently associated with elevated IgE serum levels. Additional autoimmune manifestations comprise autoimmune endocrinopathies, i.e. thyroiditis, cytopenias, including haemolytic anemia, thrombocytopenia, and neutropenia, hepatitis with positive auto-antibodies, and renal disease, either of autoimmune origin or related to the administration of immunosuppressive drugs. As a consequence of lymphoproliferation, splenomegaly and lymphadenopathy can be associated with multi-organ autoimmunity. The clinical condition can worsen with infections, although these latter can often be the consequence of immunosuppressive (IS) therapy, rather than the direct consequence of the mutation itself. In affected males, symptoms typically develop early in infancy, leading to failure to thrive and to premature death, if patients are not promptly treated [Citation6, Citation9]. The disease is rare, with less than 150 cases described worldwide in the last 10 years [Citation9].
Besides the typical early-onset severe clinical spectrum, both milder forms of the disease and IPEX-like syndromes have been described [Citation6, Citation41]. The former are characterized either by delayed onset, or better response to IS therapy and milder symptoms, whereas the latter include male and female patients presenting with autoimmune symptoms phenotypically resembling IPEX in the absence of detectable FOXP3 mutations [Citation6, Citation41]. Except for rare patients with mutations in CD25 [Citation42] or STAT5b [Citation43], in most of the cases the cause of the IPEX-like syndromes remains unknown. We have recently reported that in at least a subset of these patients autoimmunity may be caused by a quantitative defect of tTreg cells [Citation44], thus indicating Treg cells as the key contributors to disease development in both IPEX and IPEX-like syndromes.
At present, the standard treatment of IPEX syndrome is limited to supportive and replacement therapies associated with the use of multiple IS drugs, with inefficient control of autoimmunity in most of the patients. Among IS drugs, rapamycin is of particular interest since it selectively targets Teff cells and spares Treg cells, which are insensitive to mTOR inhibitors [Citation45]. So far, positive clinical results have been reported in four IPEX patients, with clinical remission in mid-long term follow-up [Citation6, Citation46, Citation47]. Although it has yet to be clarified whether rapamycin has the same effect in Treg cells from healthy donors and IPEX patients, the use of such an alternative to calcineurin inhibitors seems to be promising.
Haematopoietic stem cell transplantation (HSCT), is the only curative treatment currently available [Citation6], and the experience in transplanted IPEX patients (recently reviewed in [Citation9]) has taught that the earliest HSCT can be performed, i.e. the earliest autoimmune attack and drug-related damages are prevented, the best the outcome. This observation highlights the critical importance of early diagnosis to guarantee prompt intervention.
Both myeloablative and non-myeloablative conditioning regimens have been used (reviewed in [Citation9]). The non-myeloablative regimens may allow to reduce both post-transplant infectious complications and the toxicity of high dose chemotherapy, to which IPEX patients are very susceptible due to their generally poor clinical conditions.
On the other hand, a non-myeloablative conditioning may increase the risk of a partial chimerism, although we now know that full donor engraftment is not necessary for complete recovery, but rather the engraftment of donor Treg cells is essential to cure the disease [Citation48]. Based on the latter observation, cell/gene therapy approaches designed to selectively restore the Treg cell compartment are currently under investigation by the group of R. Bacchetta at the San Raffaele Scientific Institute of Milan, Italy, as an alternative therapeutic strategy when a suitable donor for HSCT is not available (Passerini et al., manuscript in preparation).
FOXP3 MUTATIONS AND IMMUNE DYSFUNCTIONS
Treg Cell Dysfunction
Mutations of the FOXP3 gene cause severe autoimmune disease in both humans and mice [Citation8, Citation49]. As expected based on the pattern of FOXP3 expression in the T cell compartment, the tTreg cell subset is mainly affected by FOXP3 mutations. Dysruption of FOXP3 in humans results in loss of suppressive function by Treg cells [Citation41, Citation50, Citation51], which is considered the primary cause of disease.
However, it remains unclear how the reported mutations impact on the suppressive function. The domain-specific functional impact of different mutations on FOXP3 protein has been predicted [Citation52] and a study of the model-crystal structure of the FOXP3 forkhead domain has demonstrated that specific IPEX-associated mutations affect formation of dimers without compromising FOXP3 DNA binding [Citation53]. In addition, transduction with point mutant forms of FOXP3 demonstrated incomplete reprogramming of Teff cells into Treg cells [Citation54]. These results are consistent with the observation that the degree of IPEX Treg cell functional impairment can vary from complete abrogation of suppressive function to partial dysfunction [Citation50]. Therefore, it can be hypothesized that either different FOXP3 domains are important for the establishment of suppressive function, or other genes cooperate with FOXP3 to confer appropriate suppressive function. As a result, some mutated variants of the protein could retain residual activity with incomplete impairment of Treg cell function. However, as long as the mode of action of Treg cells is undefined, the molecular mechanisms through which FOXP3 mutations impair Treg cell function will remain undefined.
In addition to the loss of suppressive function, FOXP3 mutations are associated with high instability of Treg cells, which upon inflammatory pressure convert from a regulatory to an effector (i.e. IL-17-producing) phenotype, thus potentially contributing to the autoimmune damage [Citation55]. Therefore, stable expression of wild type FOXP3 seems responsible for preservation of Treg identity.
Teff cell Dysfunction
In humans, FOXP3 is expressed transiently upon activation of Teff cells [Citation33, 34]. Based on this observation it has been hypothesized that FOXP3 mutations may also directly affect Teff cell functions. In support of this, peripheral blood mononuclear cells (PBMC) from IPEX patients have altered cytokine production, with impairment of Th1-related cytokines and relative skew to a Th2 profile [Citation50, Citation56, Citation57]. In addition, we observed an increased proportion of IL-17-producing cells in patients’ PBMC [Citation55] and in gut infiltrates (unpublished data). These data suggest that the impairment of FOXP3-dependent Teff cell function may directly contribute to the pathogenic mechanism underlying the disease.
FOXP3 Mutations and Auto-Antibodies
The impairment in the immune response regulation due to FOXP3 mutations extends beyond the T cell compartment and indirectly alters humoral immunity. FOXP3 mutations do not impact central B-cell tolerance, but rather affect the peripheral B-cell tolerance checkpoint, as demonstrated by the accumulation of autoreactive mature naïve B cells in IPEX patients [Citation58]. These results suggest an important role for Treg cells in the maintenance of peripheral B-cell tolerance in humans and highlight an additional FOXP3 mutation-dependent immune defect in IPEX syndrome.
Tissue-specific autoantibodies are detectable very early in IPEX patients, in correlation with the presence of autoimmune response to target organs. In particular, autoantibodies to enterocyte antigens, identified as the 75kDa USH1C protein, also known as harmonin, and the actin-binding 95kDa protein, also known as villin, have been reported in IPEX patients [Citation59, 60]. We have strong evidence that autoantibodies to harmonin and villin are sensitive and specific biomarkers of IPEX, which correlate with FOXP3 mutations, and can be used to differentiate IPEX from IPEX-like syndromes [Citation61]. The mechanisms responsible for harmonin and villin autoimmunization in IPEX and the role of these autoantigens in the pathological manifestations of IPEX syndrome are presently unknown, but it cannot be excluded that these antigens could be relevant molecular targets of pathogenic autoimmunity.
Type 1 Regulatory T Cells in IPEX Patients
Importantly, the presence and function of type 1 regulatory T (Tr1) cells, the major adaptive IL-10-producing Treg cell subset, are not affected by FOXP3 mutations. Indeed, functional Tr1 cells differentiate independently of FOXP3, thus suggesting that in favorable conditions, Tr1 cells could exert regulatory functions, which might compensate for the lack of tTreg dependent regulation especially in long-term surviving IPEX patients [Citation62].
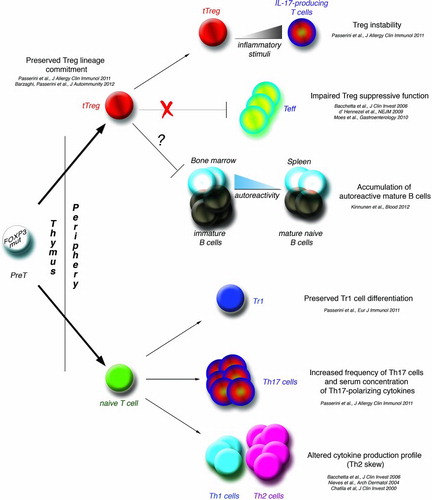
Based on the above, our current view of the pathogenesis of IPEX syndrome proposes the impairment of Treg cells as a major step, but also includes other events contributing to the maintenance of immune dysfunction, such as auto-reactive Th17 cell expansion, persistence of auto-reactive B cells with autoantibodies production, in the context of an inflammatory environment ().
FIGURE 1. Pathogenesis of IPEX syndrome. The figure summarizes our current knowledge of the immune pathways directly or indirectly affected or spared by mutations in the FOXP3 gene in humans. FOXP3mut: mutations in the FOXP3 gene; tTreg: thymus-derived regulatory T cells; Tr1: type 1 regulatory T cells; Teff: T effector cells.
NATURALLY OCCURRING TREG SELECTION IN THE THYMUS
Thymic Origin of tTreg Cells
Studies in animal models have shown that a thymus-derived specific subset of CD4+ cells was able to suppress autoimmune reactions and actively transfer tolerance [Citation63–65]. However, the discovery of tTreg cells did not come with a clear understanding of the lineage-specificity and origin of these cells. It is now well established that tTreg cells originate from the thymus and are distinct from other sets of Treg cells that can differentiate from naïve T cells in the periphery (peripherally derived (p) Treg cells) [Citation10, Citation66]. Several mechanisms have been described to contribute to tTreg cell specification in the thymus, as summarized below.
TCR/MHC Interaction
tTreg cell specification in the thymus seems to be primarily dictated by the interaction of TCR with the MHC/antigen complexes expressed by thymic epithelium. Data from transgenic mice indicate that a developing T cell is primed for Treg differentiation if the avidity of its TCR for self-antigens is higher than the avidity of conventional T cell TCR [Citation67–69]. The importance of a strong TCR signaling in tTreg differentiation has been highlighted in murine models where a defective expression of molecules downstream the TCR, such as ZAP70, LAT or Pest, results in impaired Treg development [Citation70–72]. In humans, polymorphisms in the PTPN22 gene, which encodes for a negative regulator of TCR signaling, have been linked to autoimmune conditions associated with Treg cell dysfunction, and this phenotype seems to correlate with increased PTPN22 expression and subsequent reduced TCR signaling [Citation73–76].
Two-Step Model
Based on the known mechanisms of central tolerance, medium/strong TCR engagement in the thymus should lead to T cell death by apoptosis. A mechanism that specifically rescues Treg cells from this pathway has been proposed [Citation77, 78]. According to this two-step model, recognition of MHC/antigen complexes by CD25−Foxp3− T cells with high/medium avidity with simultaneous signaling of co-stimulatory molecules, leads to up-regulation of the IL-2 receptor α chain (CD25). The resulting CD25+Foxp3− primed T cells, able to respond to IL-2 and to IL-7 (γ-chain signaling cytokines), upregulate the expression of Foxp3 and transduce anti-apoptotic signals, and are thus rescued from death and committed to Treg differentiation (CD25+Foxp3+).
Role of Cytokines
In addition to TCR and γ-chain signaling cytokines, other external stimuli have been proposed to contribute to Treg cell differentiation and Foxp3 expression in the thymus. TGFβ is known to activate Foxp3 expression in peripheral naïve T cells and to promote pTreg differentiation in conjunction with TCR signaling [Citation79, 80]. However, ablation of TGFβ receptor, or of the Foxp3 regulatory element bound by its downstream signaling molecules, severely reduced peripheral Treg cells in mice, while thymic Treg cells were barely affected [Citation81–84]. These findings suggest that TGFβ is dispensable for tTreg cell development and its action at the thymic level is currently thought to be rather pro-survival than instructing [Citation85]. On the contrary, the dendritic cell-derived cytokine thymic stromal lymphopoietin (TSLP) has been shown to promote tTreg cell differentiation, but the molecular mechanisms governing this process still need to be clarified [Citation86–90].
Costimulatory Molecules
tTreg cell development is dramatically impaired in CD28 or CD28 ligands (CD80/ CD86) knockout mice, which have reduced PKC activation and subsequent hampered NF-kB pathway [Citation91–97]. The importance of NF-kB pathway in poising Foxp3 expression has been elucidated by a recent study that shows that activated c-Rel directly binds to one of the 4 cis-regulatory non-coding regions conserved in mouse and human, and primes Foxp3 for expression (see and below) [Citation84]. On the other hand, CD28 signaling via the PI3K/Akt pathway induces Foxo1/3 phosphorilation, with consequent sequestration out of the nucleus. Foxo1/3 have been recently identified by three independent studies as key positive factors directly binding to and controlling Foxp3 and the Treg signature gene CTLA4 in murine t- and pTreg cells [Citation98–100] (see below and the review by Vent-Schmidt et al.). How the negative effect of CD28 signaling on Foxo1/3 activity can be reconciled with the positive effect of CD28 signaling on Treg cell development is still debated.
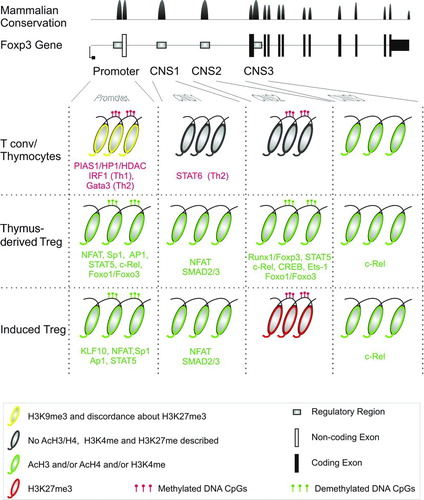
FIGURE 2. Regulation of expression of FOXP3 gene. Upper panels: FOXP3 gene structure and mammalian conservation of the DNA sequence (modified from UCSC Genome Browser). Lower panels: chromatin status at the level of Promoter, Conserved Non-coding Sequence 1 (CNS1), CNS2, and CNS3 in Thymocytes and Teff, tTreg, and i/pTreg cells. Transcription factors and chromatin modifiers binding to the depicted regions in the depicted T cell population are shown in green or red if exerting positive or negative effect on expression, respectively. For color codes and symbols of chromatin modifications see bottom panel in figure.
Thymic Development of FOXP3-Mutated tTreg Cells
Based on the phenotype of the Scurfy mouse, the natural murine mutant of foxp3, in which Foxp3-expressing cells are undetectable [Citation49], FOXP3 has been defined as the lineage determination factor for tTreg cells. According to this assumption, it has been long thought that mutations in the FOXP3 gene would drastically affect the thymic development of Treg cells in both humans and mice, resulting in the complete absence of circulating tTreg cells. Indeed, data from murine models of Foxp3 deficiency demonstrated that Foxp3 is essential for tTreg cell maintenance in the periphery, but dispensable for their development in the thymus [Citation13, Citation20]. Using reporter gene transgenic mice, a recent study showed that full Treg cell development is achieved by the concurrent establishment of specific epigenetic changes and the induction of Foxp3 expression. The two processes are independent, since the Treg cell-specific DNA methylation pattern could be established without Foxp3, whereas Foxp3 expression could occur even in the absence of the Treg-specific CpG hypomethylation pattern. Only T cells in which both events occur are developmentally set into the Treg cell lineage [Citation12]. An additional study has highlighted the role of factors other than Foxp3 in tTreg cell development. By computational network inference and experimental approaches Benoist et al. defined a set of redundant transcription factors (Eos, IRF4, Satb1, Lef1, and GATA-1) that are necessary to establish and maintain the Treg cell signature and phenotype. These data show that neither Foxp3 nor any of the aforementioned factors alone are sufficient to establish the Treg cell state, which instead needs the activation of expression patterns induced by at least two different trasncription factors [Citation101].
Similarly, we showed that in humans FOXP3 mutations do not necessarily hamper the accumulation of FOXP3 expressing cells with a Treg-like phenotype in the peripheral blood of patients [Citation6, Citation50]. Furthermore, a population of genetically imprinted Treg cells, i.e. T cells carrying specific epigenetic modifications in the regulatory regions of the FOXP3 locus, are present in peripheral blood of IPEX patients, regardless of the type or site of the mutation [Citation44, Citation55]. Therefore, while the functional impairment of FOXP3-mutated Treg cells is undisputed, the identification of circulating cells with specific demethylation of the Treg-cell-Specific-Demethylated-Region (TSDR) (see below), demonstrated that wild type FOXP3 is dispensable for thymic development of Treg cell precursors in humans [Citation44, Citation55]. The lack of functional FOXP3 impairs Treg peripheral maintenance, as demonstrated by the observation that in healthy female carriers of FOXP3 mutations [Citation102] and in transplanted IPEX patients with low peripheral donor chimerism [Citation48] only Treg cells expressing a wild type FOXP3 are detectable in peripheral blood.
Although the role of FOXP3 during Treg cell development in humans still needs to be clarified, overall these results suggest that the commitment to the Treg cell lineage can occur in the absence of functional FOXP3.
EPIGENETIC MECHANISMS CONTROLLING FOXP3 EXPRESSION IN TTREG CELLS
Epigenetics and Lineage Specification
Epigenetics collectively defines the post-translational modifications of DNA and histones (i.e. acetylation and methylation) that are not directly regulated by the underlying DNA sequence. By affecting chromatin conformation and activity of transcription factors, epigenetics plays an essential role in the development and lineage specification of adult tissues, and in particular of the T cell lineage. Recent studies have recognized altered epigenetic regulation as concurrent cause of autoimmune diseases, such as rheumatoid arthritis and SLE, as well as of allergies [Citation103, 104]. Thanks to the development of efficient and unbiased techniques to assess gene expression and histone/DNA modifications at genome level, chromatin landscape in T cells has been shown to be more dynamic than expected, showing bi-valent states and fast changes in response to external stimuli, both during differentiation and activation [Citation103, Citation105].
Cis-regulatory Elements at the FOXP3 Locus
Cis-regulatory elements have been mapped in the FOXP3 gene for binding of several transcription factors, which activate or repress expression (). Stable FOXP3 expression, which characterizes tTreg cells, appears to be maintained by epigenetic modifications, primarily composed by DNA methylation patterns and secondarily by histone marks that stabilize chromatin conformation and allow binding of the transcription factors. Conserved Non-coding Sequences (CNS) have been mapped in (i) the promoter region, (ii) and (iii) the first intron (CNS1 and CNS2), and (iv) the intron after the first coding exon (CNS3) and shown to undergo epigenetic regulation.
CNS3 Regulatory Region
H3K4me1, a marker of active enhancers, is enriched at the CNS3 region of the foxp3 locus in Treg cell and, at slightly lower levels, in naïve T cells and immature thymocytes [Citation84]. The NF-kB pathway component c-Rel, activated in response to TCR/CD28 engagement, binds the poised CNS3 region and facilitates Foxp3 transcription and Treg differentiation [Citation84]. CNS3 has been accordingly proposed as a cis-regulatory element needed for initial activation of Treg expression pattern (pioneer element). Further supporting the priming role of CNS3 in Treg determination and Foxp3 induction, a recent study has shown that the atypical inhibitor of NF-kB IkB(NS) binds CNS3 by interacting with c-Rel and p50 NF-kB and its deficiency in mice leads to impaired t/pTreg cell development [Citation106].
CNS2 Regulatory Region
While the presence of repressive histone marks on the foxp3 gene region in non-Treg cells is still debated [Citation107, 108], the DNA methylation status of promoter and CNS2 regions appears to be the main form of epigenetic control acting at the foxp3/FOXP3 locus. Several studies in both mouse and human showed that a region contained in CNS2 is demethylated in tTreg, but not in in vitro induced (i)/pTreg and Teff cells. This region, defined as TSDR, is also adorned by H3 acetylation and H3K4me3 open chromatin marks, at least in the mouse, and can act as enhancer in a luciferase reporter assay [Citation109–111]. Further studies have proposed TSDR demethylation as epigenetic modification needed for Treg lineage commitment, confirmed the tTreg specificity of this region and have shown that the transcription factors recruited to activate and maintain Foxp3-expression upon TCR/CD28 and γ-chain cytokine receptor engagement, such as CREB, Ets-1 and STAT5, can bind to this sequence only when it is demethylated [Citation112–115]. The need for CNS2 demethylation for constant Foxp3 expression was further confirmed by data showing enhanced expression of this gene upon knockdown of the DNA methyl-transferase 1 (DNMT1) in Teff cells and stabilization of iTreg phenotype upon Azacytidine-derivatives (Aza, a DNMT1 inhibitor) [Citation111, 112]. The molecular mechanism at the basis of heritable maintenance of Foxp3 expression in tTreg cells has been later revealed as a positive feedback loop mediated by the CpG demethylation dependent binding of a complex formed by Foxp3, Cbf-β, and Runx1 [Citation84, Citation116–119]. The discovery of TSDR was also important since it provided a tool to identify tTreg cells. Indeed, since FOXP3 expression is shared by both t- and pTreg cells and activated Teff cells, quantification of TSDR demethylated cells is at the moment the only method to quantify tTreg cell number, especially in humans [Citation44, Citation120]. Using this quantitative method we demonstrated that in a group of patients with IPEX symptoms the number of tTreg cells was significantly reduced in the absence of FOXP3 mutation [Citation44]. A recent study in mouse has shown that pTreg can gradually demethylate TSDR in a highly lymphopenic system [Citation12]. It still needs to be defined whether this is a general mechanism for murine and human pTreg and, thus, if TSDR demethylation differentiates between stable and unstable Treg-phenotype rather than between t and pTregs.
Promoter
A recent study has shown that the SUMO E3 ligase PIAS1 restrains Foxp3 expression by binding the foxp3 promoter in immature thymocytes and Teff cells, and maintaining a repressive chromatin status by recruiting DNMT3A/B and Heterochromatin Protein 1, eventually inducing DNA methylation and H3K9me3 modifications [Citation121]. These data suggest that during tTreg cell differentiation PIAS1 is removed from the foxp3 promoter in order to enhance accessibility and binding of the TFs induced by TCR/CD28 and IL2R signaling. Several reports have recently shown an important role of the Foxo1/3 transcription factors in controlling Foxp3 expression in both t- and pTreg cells, by directly binding to the promoter and the CNS2 region at the foxp3 locus, and regulating Treg cell differentiation [Citation99, 100, Citation122]. Foxo1 expression is directly regulated by the epigenetic regulator kap1 (also known as Trim28), whose knockout out in mouse T cells induced expansion of peripheral Treg cells in vivo and increased ability of naïve T cells to differentiate into i Treg cells [Citation123]. Kap1 has also been shown to control the expression of the PI3K antagonist PTEN in B cells and to bind to pten regulatory elements also in T cells, suggesting that it might be further involved in the Foxo1/Foxp3 axis at an upstream level ([Citation124] and F. Santoni de Sio, unpublished data).
CNS1 Regulatory Region
Foxp3 expression in naïve T cells and pTreg induction in murine cells requires TCR engagement in the presence of TGFβ [Citation79, 80]. In order to activate Foxp3 in these cells, TGFβ signaling has been demonstrated to induce histone 4 acetylation and binding of NFAT and SMAD3 to the CNS1 region [Citation80, Citation125]. While CNS2 demethylation is an exclusive requirement for tTreg cells, CNS1 region is important for pTreg cell function. By classical reverse genetic approaches two independent studies have proposed an E3 ubiquitin ligase ITCH-dependent role for Kruppel-like factor 10 (KLF10, also known as TIEG1) in the opening of the chromatin at the promoter region of foxp3 in pTreg [Citation107, Citation126]. Finally, an enhancer element upstream the foxp3 promoter has been proposed as an important regulatory region for stable Foxp3 expression in the mouse genome and found to be demethylated, bound by KLF10 and marked by histone acetylation exclusively in tTreg cells [Citation127].
Post-Transcriptional and Post-Translational Control
Although well beyond the aim of this review, two additional levels of regulation acting on Foxp3 expression need to be mentioned. The first is the post-transcriptional control of the Treg expression pattern mediated by micro RNA and the second includes the post-translational modifications FOXP3 is subject to (i.e. acetylation and phosphorilation). Several studies have indicated an important role of both processes in the control of Treg function [Citation128–130].
CONCLUSIONS
In the last ten years, since the identification of FOXP3 as key contributor to the maintenance of tTreg identity, much work has been dedicated to the identification of the molecular mechanisms leading from the induction of FOXP3 expression to the acquisition of Treg cell identity. Evidences suggest that during Treg cell development in the thymus functional FOXP3 is dispensable, whereas epigenetic remodeling is crucial for Treg lineage determination. FOXP3 expression becomes fundamental in later stages, being necessary for the peripheral maintenance of tTreg cells. It is now clear that FOXP3 plays an essential role in maintaining homeostasis of the immune system, by allowing the acquisition of full suppressive function and stability of the Treg cell lineage, regulating the production of autoantibodies, and directly modulating the expansion and function of Teff cells. Likely due to its central role in immune regulation, FOXP3 expression in Treg cells is tightly regulated, especially at the transcriptional level by the coordinated action of transcripton factors and chromatin modifying molecules. FOXP3 is therefore a complex molecule reflecting its complex task: preserve peace among the numerous players of the immune response.
ACKNOWLEDGEMENTS
The authors thank the members of the Italian Study Group of IPEX (www. ipexconsortium.org) for encouragement and support, and Dr. Philippe Begin from Stanford University, for editing the manuscript. The authors are grateful to the patients and their families for their trust and participation in our studies.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Our work is supported by the Telethon Foundation (Tele 10A4 to R.B.), the Italian Ministry of Health (Grant RF-2009-1485896 to R.B. and GR-2010-2317550 to F.R.S.d.S.), and the Seventh Framework project (FP7) of the European Community (Cell-PID to R.B.).
REFERENCES
- Al-Herz W, Bousfiha A, Casanova JL, Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2011;2:54.
- Ferraro A, Socci C, Stabilini A, Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 2011;60(11):2903–2913.
- Barreto M, Ferreira RC, Lourenco L, Low frequency of CD4+CD25+ Treg in SLE patients: a heritable trait associated with CTLA4 and TGFbeta gene variants. BMC Immunol 2009;10:5.
- Marangoni F, Trifari S, Scaramuzza S, WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med 2007;204(2): 369–380.
- Sauer AV, Brigida I, Carriglio N, Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood 2012;119(6):1428–1439.
- Gambineri E, Perroni L, Passerini L, Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol 2008;122(6):1105–1112 e1.
- Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 2009;10(7):689–695.
- Wildin RS, Ramsdell F, Peake J, X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001;27(1): 18–20.
- Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol 2012;3:211. doi: 10.3389/fimmu.2012.00211.
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299(5609):1057–1061.
- Hill JA, Feuerer M, Tash K, Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 2007;27(5):786–800.
- Ohkura N, Hamaguchi M, Morikawa H, T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 2012;37(5):785–799.
- Gavin MA, Rasmussen JP, Fontenot JD, Foxp3-dependent programme of regulatory T-cell differentiation. Nature 2007;445(7129):771–775.
- Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T. Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(-) cells. Eur J Immunol 2008;38(5):1381–1390.
- Allan SE, Alstad AN, Merindol N, Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 2008;16(1):194–202.
- Abbas AK, Benoist C, Bluestone JA, Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 2013;14(4):307–308.
- Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 2007;8(3):277–284.
- Hoffmann P, Boeld TJ, Eder R, Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 2009;39(4):1088–1097.
- d'Hennezel E, Yurchenko E, Sgouroudis E, Single-cell analysis of the human T regulatory population uncovers functional heterogeneity and instability within FOXP3+ cells. J Immunol 2011;186(12):6788–6797.
- Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 2007;445(7129):766–770.
- Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol 2010;22(5): 575–582.
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009;10(9):1000–1007.
- Voo KS, Wang YH, Santori FR, Identification of IL-17-producing FOXP3 +regulatory T cells in humans. Proc Natl Acad Sci USA 2009;106(12):4793–4798.
- Beriou G, Costantino CM, Ashley CW, IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 2009;113(18):4240–4249.
- Ayyoub M, Deknuydt F, Raimbaud I, Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA 2009;106(21):8635–8640.
- Koenen HJ, Smeets RL, Vink PM, Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008;112(6):2340–2352.
- Hansmann L, Schmidl C, Kett J, Dominant Th2 differentiation of human regulatory T cells upon loss of FOXP3 expression. J Immunol 2012;188(3):1275–1282.
- Rubtsov YP, Niec RE, Josefowicz S, Stability of the regulatory T cell lineage in vivo. Science 2010;329(5999):1667–1671.
- Miyao T, Floess S, Setoguchi R, Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012;36(2): 262–275.
- Hori S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol 2011;32(7):295–300.
- Hori S. The Foxp3 interactome: a network perspective of T(reg) cells. Nat Immunol 2012;13(10): 943–945.
- Duhen T, Duhen R, Lanzavecchia A, Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012;119(19):4430–4440.
- Allan SE, Crome SQ, Crellin NK, Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 2007;19(4):345–354.
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 2007;110(8):2983–2990.
- McMurchy AN, Gillies J, Gizzi MC, A novel function for FOXP3 in humans: intrinsic regulation of conventional T cells. Blood 2013;121(8):1265–1275.
- Merlo A, Casalini P, Carcangiu ML, FOXP3 expression and overall survival in breast cancer. J Clin Oncol 2009;27(11):1746–1752.
- Zuo T, Wang L, Morrison C, FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell 2007;129(7):1275–1286.
- Zuo T, Liu R, Zhang H, FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest 2007;117(12):3765–3773.
- Ebert LM, Tan BS, Browning J, The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res 2008;68(8):3001–3009.
- Wang L, Liu R, Li W, Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell 2009;16(4):336–346.
- Moes N, Rieux-Laucat F, Begue B, Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology 2010;139(3):770–778.
- Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA 1997;94(7):3168–3171.
- Cohen AC, Nadeau KC, Tu W, Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol 2006;177(5):2770–2774.
- Barzaghi F, Passerini L, Gambineri E, Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J Autoimmun 2012;38(1):49–58.
- Battaglia M, Stabilini A, Migliavacca B, Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 2006;177(12):8338–8347.
- Bindl L, Torgerson T, Perroni L, Successful use of the new immune-suppressor sirolimus in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome). J Pediatr 2005;147(2):256–259.
- Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol 2008;28(5):581–587.
- Seidel MG, Fritsch G, Lion T, Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood 2009;113(22):5689–5691.
- Brunkow ME, Jeffery EW, Hjerrild KA, Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001;27(1): 68–73.
- Bacchetta R, Passerini L, Gambineri E, Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 2006;116(6):1713–1722.
- d'Hennezel E, Ben-Shoshan M, Ochs HD, FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N Engl J Med 2009;361(17):1710–1713.
- d'Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo C. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 2012;49(5): 291–302.
- Bandukwala HS, Wu Y, Feuerer M, Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells. Immunity 2011;34(4):479–491.
- McMurchy AN, Gillies J, Allan SE, Point mutants of forkhead box P3 that cause immune dysregulation, polyendocrinopathy, enteropathy, X-linked have diverse abilities to reprogram T cells into regulatory T cells. J Allergy Clin Immunol 2010;126(6):1242–1251.
- Passerini L, Olek S, Di Nunzio S, Forkhead box protein 3 (FOXP3) mutations lead to increased TH17 cell numbers and regulatory T-cell instability. J Allergy Clin Immunol 2011;128(6): 1376–1379 e1.
- Nieves DS, Phipps RP, Pollock SJ, Dermatologic and immunologic findings in the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Arch Dermatol 2004;140(4): 466–472.
- Chatila TA, Blaeser F, Ho N, JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 2000;106(12):R75–R81.
- Kinnunen T, Chamberlain N, Morbach H, Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 2013;121(9):1595–1603.
- Kobayashi I, Kubota M, Yamada M, Autoantibodies to villin occur frequently in IPEX, a severe immune dysregulation, syndrome caused by mutation of FOXP3. Clin Immunol 2011;141(1):83–89.
- Kobayashi I, Imamura K, Kubota M, Identification of an autoimmune enteropathy-related 75-kilodalton antigen. Gastroenterology 1999;117(4):823–830.
- Lampasona V, Passerini L, Barzaghi F, Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One 2013;8(11):e78664. doi: 10.1371/ journal.pone.0078664.
- Passerini L, Di Nunzio S, Gregori S, Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome. Eur J Immunol 2011;41(4):1120–1131.
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med 1985;161(1):72–87.
- Sakaguchi S, Sakaguchi N, Asano M, Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155(3):1151–1164.
- Ohki H, Martin C, Corbel C, Tolerance induced by thymic epithelial grafts in birds. Science 1987;237(4818):1032–1035.
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25 +regulatory T cells. Nat Immunol 2003;4(4):330–336.
- Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 2006;25(2):249–259.
- Wong J, Obst R, Correia-Neves M, Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol 2007;178(11):7032–7041.
- Hsieh CS, Liang Y, Tyznik AJ, Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 2004;21(2):267–277.
- Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25 +regulatory T cell development. J Exp Med 2006;203(1):119–129.
- Tanaka S, Maeda S, Hashimoto M, Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol 2010;185(4):2295–2305.
- Maine CJ, Hamilton-Williams EE, Cheung J, PTPN22 alters the development of regulatory T cells in the thymus. J Immunol 2012;188(11):5267–5275.
- Todd JA, Walker NM, Cooper JD, Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39(7):857–864.
- Criswell LA, Pfeiffer KA, Lum RF, Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 2005;76(4):561–571.
- Bottini N, Musumeci L, Alonso A, A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004;36(4):337–338.
- Zhang J, Zahir N, Jiang Q, The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet 2011;43(9):902–907.
- Burchill MA, Yang J, Vang KB, Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 2008;28(1):112–121.
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity 2008;28(1):100–111.
- Chen W, Jin W, Hardegen N, Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198(12):1875–1886.
- Tone Y, Furuuchi K, Kojima Y, Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 2008;9(2):194–202.
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 2006;25(3):455–471.
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006;25(3):441–454.
- Liu Y, Zhang P, Li J, A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008;9(6):632–640.
- Zheng Y, Josefowicz S, Chaudhry A, Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010;463(7282):808–812.
- Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 2010;32(5):642–653.
- Watanabe N, Wang YH, Lee HK, Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005;436(7054):1181–1185.
- Hanabuchi S, Ito T, Park WR, Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol 2010;184(6):2999–3007.
- Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood 2010;115(26):5366–5375.
- Proietto AI, van Dommelen S, Zhou P, Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA 2008;105(50):19869–19874.
- Roman E, Shino H, Qin FX, Liu YJ. Cutting edge: hematopoietic-derived APCs select regulatory T cells in thymus. J Immunol 2010;185(7):3819–3823.
- Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol 2004;173(8): 5028–5035.
- Salomon B, Lenschow DJ, Rhee L, B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000;12(4):431–440.
- Tang Q, Henriksen KJ, Boden EK, Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25 +regulatory T cells. J Immunol 2003;171(7):3348–3352.
- Schmidt-Supprian M, Tian J, Grant EP, Differential dependence of CD4+CD25 +regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci USA 2004;101(13):4566–4571.
- Medoff BD, Sandall BP, Landry A, Differential requirement for CARMA1 in agonist-selected T-cell development. Eur J Immunol 2009;39(1):78–84.
- Barnes MJ, Krebs P, Harris N, Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol 2009;7(3):e51.
- Long M, Park SG, Strickland I, Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009;31(6):921–931.
- Kerdiles YM, Stone EL, Beisner DR, Foxo transcription factors control regulatory T cell development and function. Immunity 2010;33(6):890–904.
- Harada Y, Elly C, Ying G, Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med 2010;207(7):1381–1391.
- Ouyang W, Liao W, Luo CT, Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 2012;491(7425):554–559.
- Fu W, Ergun A, Lu T, A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol 2012;13(10):972–980.
- Di Nunzio S, Cecconi M, Passerini L, Wild-type FOXP3 is selectively active in CD4+CD25(hi) regulatory T cells of healthy female carriers of different FOXP3 mutations. Blood 2009;114(19):4138–4141.
- Cuddapah S, Barski A, Zhao K. Epigenomics of T cell activation, differentiation, and memory. Curr Opin Immunol 2010;22(3):341–347.
- Kanno Y, Vahedi G, Hirahara K, Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol 2012;30:707–731.
- Mohammad HP, Baylin SB. Linking cell signaling and the epigenetic machinery. Nat Biotechnol 2010;28(10):1033–1038.
- Schuster M, Glauben R, Plaza-Sirvent C, IkappaB(NS) protein mediates regulatory T cell development via induction of the Foxp3 transcription factor. Immunity 2012;37(6):998–1008.
- Xiong Y, Khanna S, Grzenda AL, Polycomb antagonizes p300/CREB-binding protein-associated factor to silence FOXP3 in a Kruppel-like factor-dependent manner. J Biol Chem 2012;287(41):34372–34385.
- Wei G, Wei L, Zhu J, Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009;30(1): 155–167.
- Baron U, Floess S, Wieczorek G, DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 2007;37(9):2378–2389.
- Floess S, Freyer J, Siewert C, Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007;5(2):e38.
- Polansky JK, Kretschmer K, Freyer J, DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008;38(6):1654–1663.
- Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 2007;204(7):1543–1551.
- Polansky JK, Schreiber L, Thelemann C, Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl) 2010;88(10):1029–1040.
- Zorn E, Nelson EA, Mohseni M, IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006;108(5):1571–1579.
- Toker A, Engelbert D, Garg G, Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol 2013;190(7):3180–3188.
- Ono M, Yaguchi H, Ohkura N, Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 2007;446(7136):685–689.
- Rudra D, Egawa T, Chong MM, Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol 2009;10(11):1170–1177.
- Kitoh A, Ono M, Naoe Y, Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity 2009;31(4):609–620.
- Bruno L, Mazzarella L, Hoogenkamp M, Runx proteins regulate Foxp3 expression. J Exp Med 2009;206(11):2329–2337.
- Wieczorek G, Asemissen A, Model F, Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 2009;69(2):599–608.
- Liu B, Tahk S, Yee KM, The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 2010;330(6003):521–525.
- Ouyang W, Beckett O, Ma Q, Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 2010;11(7):618–627.
- Santoni de Sio FR, Barde I, Offner S, KAP1 regulates gene networks controlling T-cell development and responsiveness. FASEB J 2012;26(11):4561–4575.
- Santoni de Sio FR, Massacand J, Barde I, KAP1 regulates gene networks controlling mouse B-lymphoid cell differentiation and function. Blood 2012;119(20):4675–4685.
- Vaeth M, Schliesser U, Muller G, Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3 +regulatory T cells. Proc Natl Acad Sci USA 2012;109(40):16258–16263.
- Venuprasad K, Huang H, Harada Y, The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol 2008;9(3):245–253.
- Lal G, Zhang N, van der Touw W, Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol 2009;182(1):259–273.
- Cobb BS, Hertweck A, Smith J, A role for Dicer in immune regulation. J Exp Med 2006;203(11):2519–2527.
- Liston A, Lu LF, O'Carroll D, Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med 2008;205(9):1993–2004.
- Zhang H, Xiao Y, Zhu Z, Immune regulation by histone deacetylases: a focus on the alteration of FOXP3 activity. Immunol Cell Biol 2012;90(1):95–100.
