Abstract
Renal ischemia/reperfusion (I/R) occurs during shock and transplant procedures, greatly affecting outcome. A definitive treatment has not been found. One of the pathophysiological bases of renal I/R injury is the activation of the transcription factor nuclear factor-kappaB (NF-KappaB). We studied the effects of sulfasalazine (SFZ), a NF-kappaB inhibitor, over renal injury in a bilateral renal I/R model in rats. Ten male Wistar rats were subjected to bilateral renal I/R for 45 min followed by 24 h of reperfusion. Half of these received 100 mg/kg SFZ orally before the induction of I/R, while the others received only saline. Five rats served as sham-operated controls. At the end of the reperfusion period, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), P-selectin, tumor necrosis factor-alpha (TNF-alpha), intracellular adhesion molecule-1 (ICAM-1), and endothelin-1 (ET-1) concentrations were determined in serum, and renal samples were taken for histological evaluation. After renal I/R, AST, LDH, BUN, TNF-alpha, ICAM-1, and ET-1 serum levels were significantly increased, and tubules were severely damaged on histological analysis, compared to sham controls. SFZ treatment reduced the AST, LDH, BUN, TNF-alpha, and ET-1 elevations, as well as the tubular damage, induced by renal I/R. Serum ICAM-1 and P‐selectin were unchanged. These results show that SFZ has a protective effect over renal IR injury. The modulation of adhesion molecules probably does not play a part in these effects, but TNF-alpha and ET-1 modulation could be partly responsible for the effects we observed.
INTRODUCTION
Renal ischemia occurs during shock and aortic surgical procedures. Renal transplantation also invariably involves a period of ischemia and subsequent reperfusion.Citation[1] Moreover, ischemia-reperfusion (I/R) injury is a determinant factor that can alter graft and patient survival. The pathophysiology of renal I/R is very complex.Citation[2] Hypoxia induces an inflammatory response mediated by cytokines, chemokines, adhesion molecules, leukocyte infiltration, and free radicals, among others.Citation[3] Reperfusion causes additional damage and augments this initial inflammatory response. Transcription factors are also activated in epithelial and inflammatory cells. One of these factors, nuclear factor kappa-B (NF-KappaB), is thought to be one of the primary orchestrators of the inflammatory cascade.Citation[4] NF-KappaB is activated and upregulated during renal I/R and is capable of promoting the transcription of cytokines such as tumor necrosis factor alpha (TNF-alpha), chemokines, and adhesion molecules, and can mediate apoptosis in damaged cells.Citation[5–7] The search for nephroprotective agents is an active area of research, with many different modalities being investigated.Citation[8] The therapeutic inhibition of NF-KappaB is a promising strategy to reduce renal I/R injury.Citation[9]
The endothelium is activated as a response to I/R-induced microvascular injury.Citation[10] Microvascular alterations consist of unbalances between vasoactive mediators, increased vascular permeability, and inflammatory mediators produced by endothelial cells.Citation[11,Citation12] Endothelium-leukocyte interactions are mediated by an increase in adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) and P-selectin.Citation[13] The production of endothelin-1 (ET-1) is also increased in endothelial cells during renal I/R, a mediator that promotes further dysfunction.Citation[14] NF‐KappaB has also been shown to participate in the regulation of ET-1 pathways during renal I/R.Citation[15]
The inhibition of NF-KappaB with decoy ligodeoxynucleotides and dithiocarbamates has been demonstrated to have beneficial effects over renal I/R in rodent experimental models.Citation[16,Citation17] Furthermore, a recent study found that blocking NF-kappaB with a genetic negative regulator could inhibit the endothelial cell production of adhesion molecules and inflammatory cytokines during renal I/R in the rat.Citation[18] Sulfasalazine (SFZ) is an anti-inflammatory drug used in the treatment of arthritis and inflammatory bowel disease, the main mechanism of action for which is also the inhibition of NF-KappaB.Citation[19] SFZ has shown protection from I/R injury in the heartCitation[20] and liverCitation[21] in rodent experimental models. To the best of our knowledge, the effects of SFZ over renal I/R injury have not been studied before. In this study, we investigated the effects of SFZ over I/R-induced renal injury, dysfunction, circulating levels of the adhesion molecules P-selectin and ICAM-1, ET-1, as well as the pro-inflammatory cytokine TNF-alpha.
MATERIAL AND METHODS
Animal Procedures
Animal procedures were performed in accordance with the proper use and care of laboratory animals and were approved by the ethics committee of our institution. Experiments were performed on 15 male Wistar rats weighing 200–250 g. Animals were maintained under standard conditions, such as stable room temperature (24 ± 3°), a 12 h light/12 h dark cycle, and access to commercial rat pellets and water ad libitum.
Animal Model
Briefly, after pentobarbital sodium anesthesia (Anestesal, Pfizer Inc, Mexico) (35 mg/kg, i.p), midline laparotomy was performed, and the renal pedicle on both sides were identified and isolated. Animals were placed under heating lamp in order to preserve core body temperature at (37°C). To induce bilateral renal ischemia, renal pedicles were occluded using non-traumatic microvascular clamps simultaneously for 45 minutes. This procedure effectively occluded both renal vein and artery, and was confirmed by changes in renal coloration and loss of pulsations. After the ischemic period, the clamps were removed, the abdominal wall and skin was sutured using 3-0 silk sutures, and the animals were then left to recover to allow 24 hours of reperfusion.
Rats were divided in three groups (n = 5). The first group received only sham surgery (sham), where laparotomy was performed but kidneys were only manipulated but not made ischemic. IR group was subjected to bilateral I/R as described above, receiving only saline via gavage. Group IR+SFZ received 100 mg/kg of sulfasalazine (Azulfidine, Pfizer inc., Mexico) via gavage 15 minutes before being subjected to bilateral renal I/R as described above.
Serum Lactate Dehydrogenase (LDH), Aspartate Aminotransferase (AST), and Blood Urea Nitrogen (BUN)
At the end of the reperfusion period, 3 ml of blood was obtained from each rat and left to clot for serum acquisition. Serum levels of LDH, AST and BUN were determined by standard biochemical automated methods, using DT6011 and DTSC11 analyzers (System Vitros Chemical, Johnson and Johnson, Langhorne, Pennsylvania, USA).
Morphological Examination
Immediately after concluding the reperfusion period, rats were sacrificed by exsanguination from the aorta, and bilateral kidney tissue samples were obtained and fixed in 10% neutral buffered formalin overnight. Samples were then embedded in paraffin, and 4 micrometer-thick sections were stained with hematoxylin and eosin (H/E) and examined under light microscope by a blinded pathologist. Tubular damage was assessed by considering tubular cell necrosis, tubular dilatation, proteinaceous cast formation, and cellular sloughing, and the following values were assigned: 0: none, 1: < 5%, 2: 5–25%, 3: 25–50%, 4: 50–75%, and 5> 75% of area affected.
Serum Levels of ET-1, P-selectin, TNF-alpha, and ICAM-1
Serum concentrations of ET-1 were determined using a rat ET-1 EIA kit (Immuno-Biological Laboratories, Japan). Serum concentrations of soluble ICAM-1 were determined using a quantikine rat sICAM-1 ELISA kit (R & D Systems, Minneapolis, Minnesota, USA). Serum concentrations of P-selectin were determined using a quantikine rat P-selectin ELISA kit (R& D Systems). Serum concentrations of TNF-alpha were determined using a rat TNF-alpha Elisa kit (PeproTech, Mexico).
Statistical Analysis
SPSS 11.0 statistical software (SPSS Inc. Software, Chicago, Illinois, USA) was used to analyze data using a one-way analysis of variance (ANOVA) and with Tukey-Kramer post-hoc test so as to determine comparison between groups and differences between groups, respectively. All values are expressed as mean ± SD, and p < 0.05 was considered statistically significant.
RESULTS
Serum Lactate Dehydrogenase (LDH), Aspartate Aminotransferase (AST), and Blood Urea Nitrogen (BUN)
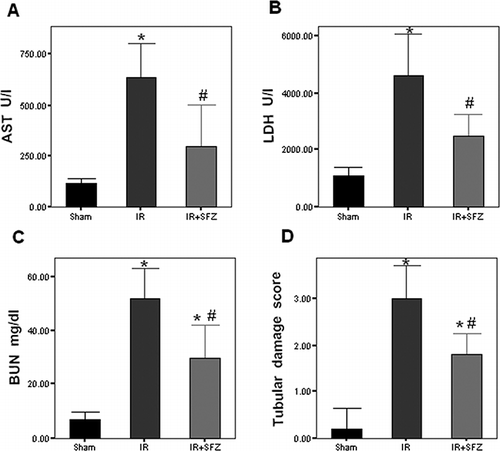
Serum LDH and AST are unspecific markers for cellular injury or necrosis. Sham group had AST and LDH serum levels of 114.2 ± 23.5 and 1083 ± 319.1 U/I, respectively. Renal IR caused a significant increase in both AST and LDH levels (637.6 ± 162.56 and 4594.4 ± 1460.9 U/I, respectively, p < 0.05; see ). SFZ treatment was able to significantly reduce both LDH and AST elevations (294 ± 210.21 and 2500 ± 755.15 U/I, respectively, p < 0.05 vs. IR; see ). Serum BUN levels, a measurement of renal function, in the sham group were 7 ± 2.74 mg/dL. BUN levels were significantly increased in IR group (52 ± 10.95 mg/dL, p < 0.05; see ). In the IR+SFZ group, BUN levels were significantly lower compared to IR group (30 ± 12.5 mg/dL, p < 0.05; see ).
Figure 1. AST, LDH, BUN, and tubular damage score. After renal I/R, AST (A), LDH (B), and BUN (C) serum levels were significantly increased compared to sham group, but these elevations were diminished by SFZ treatment. IR group showed a significantly increased tubular damage (D) score compared to sham group. Group IR+SFZ showed a significantly lower damage score when compared to IR group. *p < 0.05 vs. sham group. #p < 0.05 vs. IR group.
Histological Examination
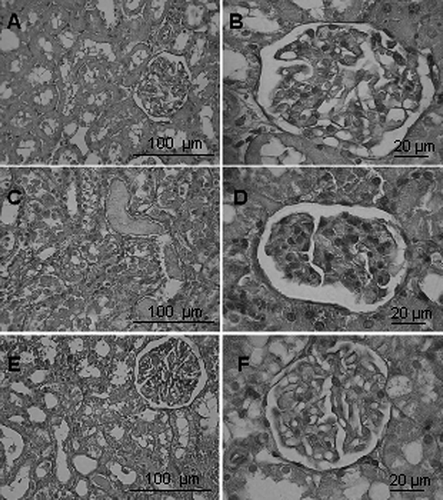
After I/R, kidneys in the IR group showed marked tubular necrosis, cast formation, and tubular cell sloughing as well as inflammatory cell infiltration (see ). Cells in glomeruli had condensed nuclei, and there was an increased bowman space. These changes were also present in IR+SFZ group, but to a lesser degree. Tubular damage score was 0.2 ± 0.45 for the sham group. In the IR group, the score was significantly increased to 3 ± 0.7. In the IR+SFZ group, the score was moderately, but significantly, lower, at 1.8 ± 0.45 (p < 0.05; see ).
Figure 2. H&E stained kidney sections. A–B: sham group showing normal tubular and glomerular structure. C–D: In IR group, tubular cell sloughing, nuclear condensation, cast formation, and tubular cell necrosis were found, as was bowman space enlargement. E–F: SFZ pretreatment diminished these alterations in group IR+SFZ.
Serum Concentrations of P-selectin, ICAM-1, ET-1, and TNF-alpha
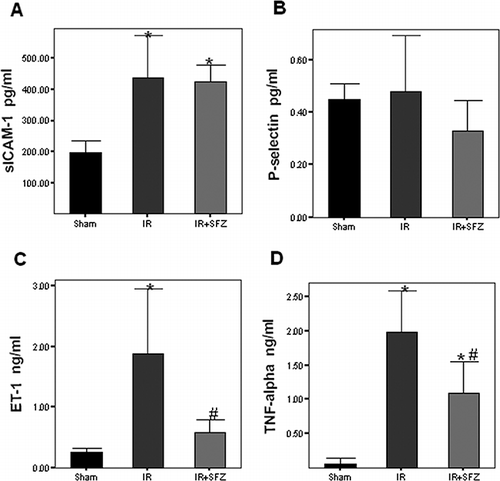
Serum P-selectin levels in sham group were 0.45 ± 0.06 pg/mL. Neither the IR nor IR+SFZ group had significant changes in P-selectin levels compared to controls (0.48 ± 0.22 pg/mL and 0.33 ± 0.11, respectively; see ). Soluble ICAM-1 serum concentrations were 197 ± 38.87 pg/mL in sham group. Renal I/R significantly increased soluble ICAM-1 levels in both the IR and IR+SFZ groups, but SFZ pretreatment failed to alter these changes, as no statistical difference was found between these two groups (438.88 ± 137.5 pg/mL and 424.4 ± 55.89 pg/mL, respectively; see ). ET-1 serum levels were 0.25 ± 0.06 ng/mL in the sham group, and in the IR group, ET-1 levels increased to 1.89 ± 1.04 ng/mL. SFZ treatment was able to abolish the ET-1 elevation induced by IR (IR+SFZ: 0.57 ± 0.2 ng/mL, p < 0.05 vs. IR; see ). Finally, TNF-alpha serum levels in sham group were undetectable by our assay. However, in the IR group, TNF-alpha levels rose to 1.99 ± 0.59 ng/mL, and were significantly reduced by SFZ treatment (IR+SFZ: 1.08 ± 0.47 ng/mL, p < 0.05 vs. IR; see ).
Figure 3. Serum soluble ICAM-1, P-selectin, ET-1, and TNF-alpha concentrations. ICAM-1 levels were significantly increased after renal I/R compared to sham group, but SFZ was unable to alter these values (A). P-selectin levels remained unchanged in both I/R groups compared to controls (B). After renal I/R, both ET-1 (C) and TNF-alpha (D) serum levels were significantly increased compared to sham group, and SFZ pretreatment was able to diminish these alterations. *p < 0.05 vs. sham group. #p < 0.05 vs. IR group.
DISCUSSION
Many of the pathophysiological events that culminate in the severe tubular and glomerular injury induced by renal I/R are mediated by the transcription factor NF-kappaB. NF-kappaB activation in renal endothelial and epithelial cells promotes the production of proinflammatory cytokines, chemokines, and adhesion molecules, which contribute to tissue damage and leukocyte infiltration.Citation[7,Citation18] Part of the mechanisms affecting cell survival after ischemic injury in the kidney are also regulated by NF‐kappaB by promoting the expression of genes that control inflammation, the immune response, cell death, and proliferation.Citation[4,Citation22] NF-kappaB has also been implicated in graft rejection after organ transplantation, and its role in I/R injury is an active area of research.Citation[8] Moreover, the inhibition of NF-kappaB with SFZ has proven to protect the kidney form inflammatory injury in animal models of gentamicin toxicity and ureteral obstruction.Citation[23,Citation24] Other molecular and pharmacological methods of inhibiting NF‐kappaB have shown beneficial effects over renal I/R histopathological, biochemical, and functional alterations in different animal models.Citation[16–18] We found that 45 min of bilateral renal ischemia followed by 24 h reperfusion caused serum AST, LDH, and BUN elevations. Elevated AST levels, an enzyme present in renal tubular cells, are thought to reflect renal and not concomitant liver damage in renal IR models, where it has been shown that ALT, a more specific liver enzyme, is not elevated.Citation[25] LDH is a non-specific marker of cellular injury and necrosis, but LDH elevations after renal IR have consistently been able to reflect organ tissue damage accurately.Citation[26] In our study, SFZ treatment was able to ameliorate these biochemical alterations, suggesting renal tissue and functional protection. Furthermore, histological analysis confirmed that SFZ was able to exert a moderate but significant protection against I/R-induced tubular damage.
Leukocytes recruited during reperfusion are important mediators of renal I/R injury, and the endothelium-leukocyte interactions directly responsible for these processes are themselves mediated by the expression of adhesion molecules, such as ICAM-1 and P-selectin.Citation[27] Increased expression of ICAM-1 and P-selectin has been demonstrated in the endothelium of capillaries in both cortex and medulla after renal experimental I/R.Citation[28] Support of the important role of these adhesion molecules in the pathophysiology of renal I/R comes from studies that demonstrate that blocking them markedly reduces I/R-induced injury and dysfunction.Citation[29] In an animal model of heart transplant, SFZ could increase graft survival and decrease tissue expression of ICAM-1 and P-selectin.Citation[20] SFZ has also been shown to inhibit ICAM-1 and P-selectin expression in human intestinal mucosa and reduce the serum levels of soluble fractions of these molecules in patients with rheumatoid arthritis.Citation[30,Citation31] In agreement with the reported literature, we found that ICAM-1 serum levels are increased after renal I/R, but also that SFZ at 100 mg/kg had no effect on this parameter. Moreover, ICAM-1 levels were not correlated with injury severity in our study, making the inhibition of ICAM-1 an unlikely mechanism of action of SFZ in protection from renal I/R. In sharp contrast, P-selectin serum levels remained unchanged in both I/R groups compared to controls. This could be explained by the time course of P-selectin activity during renal I/R. P-selectin is thought to be one of the earliest adhesion molecules expressed, after only 20 minutes of reperfusion, reaching peak levels at 5 hours and decreasing at 10 hours after renal I/R in one rodent model.Citation[32] The reperfusion time in our study was considerably longer, at 24 hours. More research is needed to establish if SFZ can indeed alter adhesion molecule levels during I/R, including serum time course studies and tissue expression of these molecules.
Endothelins are vasoactive regulatory peptides, produced in the endothelium, liver, and lungs, that are thought to participate in the pathogenesis of I/R injury in various organs.Citation[33] ET-1 is overproduced after renal I/R in reaction to hypoxia and re-oxygenation, causing deleterious effects over renal perfusion and leading to further damage.Citation[34] Blocking ET-1 receptors has proven to be an effective method in reducing the hemodynamic, functional, and histological alterations caused by renal I/R.Citation[35] Moreover, a role for NF-kappaB in ET-1 signaling during I/R has been hypothesized. Pathways that lead from NF-kappaB activation to the regulation of ET-1 expression and from ET-1 production to increased NF-kappaB activity have been found in different models.Citation[36,Citation37] Molecular inhibition of NF-kappaB was able to diminish ET-1 elevations in at least one rodent model of renal I/R.Citation[17] In our study, we found that ET-1 levels were increased after renal I/R, and that SFZ pretreatment was able to abolish this alteration. This supports the idea of a role for NF-kappaB in ET-1 signaling, and suggests a possible mechanism by which SFZ could exert its protective effects over renal I/R injury and functional alterations. Further research is needed to establish this possibility directly, and to clarify the mechanisms involved.
The pro-inflammatory cytokine TNF-alpha is produced in the kidney as a response to, and is implicated in the pathophysiology of, renal I/R injury and dysfunction.Citation[38] TNF-alpha mediates cell death, inflammatory cell infiltration, and fibrin deposition, and induces vasoconstriction after renal I/R.Citation[39] The inhibition of TNF-alpha with monoclonal antibodies, TNF-binding protein, and pentoxifylline have all proven to be effective in reducing renal I/R injury,Citation[38,Citation40,Citation41] and studies have strongly linked the activation of NF-kappaB as one of the main signals that promote TNF-alpha production after renal I/R.Citation[5,Citation39] Moreover, clinical studies have demonstrated the ability of SFZ to reduce serum TNF-alpha levels in patients with rheumatoid arthritis.Citation[42] SFZ has been shown to inhibit TNF-alpha by blocking the NF-kappaB pathway, but also directly, by competitive binding to soluble TNF-alpha, and by preventing its release by inflammatory cells.Citation[43] In our experimental model, we found that serum TNF-alpha levels were increased after renal I/R, and that SFZ could reduce these elevations. This effect could also be responsible for the beneficial effects of SFZ over renal I/R injury that we observed in our study.
In conclusion, we showed that SFZ treatment could reduce the serum biochemical markers of renal injury and function, the ET-1 and TNF-alpha elevations, as well as the tubular damage induced by bilateral renal I/R. SFZ's inhibition of TNF-alpha and ET-1 levels could partly explain our results. Moreover, SFZ did not appear to act through the inhibition of adhesion molecules.
ACKNOWLEDGMENTS
The authors would like to thank MVZ Jose Luis Vazquez Juarez for providing animals and ensuring their care. We would also like to thank the staff at the Unidad de Hígado, UANL, for technical assistance.
The authors also report no conflicts of interest, and alone are responsible for the content and writing of the paper.
REFERENCES
- Böhmová R, Viklický O. Renal ischemia-reperfusion injury: An inescapable event affecting kidney transplantation outcome. Folia Microbiol (Praha). 2001;46:267–276.
- Toledo-Pereyra LH, Toledo AH, Walsh J, Lopez-Neblina F. Molecular signaling pathways in ischemia/reperfusion. Exp Clin Transplant. 2004;2:174–177.
- Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13.
- Nichols TC. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104.
- Donnahoo KK, Meldrum DR, Shenkar R, Chung CS, Abraham E, Harken AH. Early renal ischemia, with or without reperfusion, activates NFkappaB and increases TNF-alpha bioactivity in the kidney. J Urol. 2000;163:1328–1332.
- Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747.
- Meldrum KK, Hile K, Meldrum DR, Crone JA, Gearhart JP, Burnett AL. Simulated ischemia induces renal tubular cell apoptosis through a nuclear factor-kappaB dependent mechanism. J Urol. 2002;168:248–252.
- Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: A histopathologic study. Ren Fail. 2009;31:70–74.
- Tsoulfas G, Geller DA. NF-kappaB in transplantation: Friend or foe? Transpl Infect Dis. 2001;3:212–219.
- Takano T, Brady HR. The endothelium in glomerular inflammation. Curr Opin Nephrol Hypertens. 1995;4:277–286.
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:102–107.
- Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502–516.
- Dragun D, Haller H. Diapedesis of leukocytes: Antisense oligonucleotides for rescue. Exp Nephrol. 1999;7:185–192.
- Kurata H, Takaoka M, Kubo Y, . Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol. 2005;517:232–239.
- Ohkita M, Takaoka M, Shiota Y, Nojiri R, Matsumura Y. Nitric oxide inhibits endothelin-1 production through the suppression of nuclear factor kappa B. Clin Sci (Lond). 2002;103 (Suppl.) 48:68S–71S.
- Chatterjee PK, di Villa Bianca RD, Sivarajah A, McDonald MC, Cuzzocrea S, Thiemermann C. Pyrrolidine dithiocarbamate reduces renal dysfunction and injury caused by ischemia/reperfusion of the rat kidney. Eur J Pharmacol. 2003;482:271–280.
- Cao CC, Ding XQ, Ou ZL, . In vivo transfection of NF-kappaB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int. 2004;65:834–845.
- Lutz J, Luong le A, Strobl M, . The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med. 2008;86:1329–1339.
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174.
- Feeley BT, Park AK, Hoyt EG, Robbins RC. Sulfasalazine inhibits reperfusion injury and prolongs allograft survival in rat cardiac transplants. J Heart Lung Transplant. 1999;18:1088–1095.
- Matsui N, Kasajima K, Hada M, . Inhibiton of NF-kappaB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci. 2005;30:103–110.
- Loverre A, Ditonno P, Crovace A, . Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: Differential modulation by rapamycin. J Am Soc Nephrol. 2004;15:2675–2686.
- Demirbilek S, Emre MH, Aydin EN, . Sulfasalazine reduces inflammatory renal injury in unilateral ureteral obstruction. Pediatr Nephrol. 2007;22:804–812.
- Tugcu V, Ozbek E, Tasci AI, . Selective nuclear factor kappa-B inhibitors, pyrolidium dithiocarbamate and sulfasalazine, prevent the nephrotoxicity induced by gentamicin. BJU Int. 2006;98:680–686.
- Chatterjee PK, Cuzzocrea S, Brown PA, . Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673.
- Tuğtepe H, Sener G, Biyikli NK, . The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regul Pept. 2007;140:101–108.
- Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468.
- Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818.
- Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54.
- Pooley N, Ghosh L, Sharon P. Up-regulation of E-selectin and intercellular adhesion molecule-1 differs between Crohn’s disease and ulcerative colitis. Dig Dis Sci. 1995;40:219–225.
- Veale DJ, Maple C, Kirk G, McLaren M, Belch JJ. Soluble cell adhesion molecules—P-selectin and ICAM-1, and disease activity in patients receiving sulphasalazine for active rheumatoid arthritis. Scand J Rheumatol. 1998;27:296–299.
- Zizzi HC, Zibari GB, Granger DN, . Quantification of P‐selectin expression after renal ischemia and reperfusion. J Pediatr Surg. 1997;32:1010–1013.
- Battistini B, Dussault P. The many aspects of endothelins in ischemia-reperfusion injury: Emergence of a key mediator. J Invest Surg. 1998;11:297–313.
- Ajis A, Bagnall NM, Collis MG, Johns EJ. Effect of endothelin antagonists on the renal haemodynamic and tubular responses to ischaemia-reperfusion injury in anaesthetised rats. Exp Physiol. 2003;88:483–490.
- Jerkić M, Miloradović Z, Jovović D, . Relative roles of endothelin-1 and angiotensin II in experimental post-ischaemic acute renal failure. Nephrol Dial Transplant. 2004;19:83–94.
- Müller DN, Dechend R, Fiebeler A, Park JK, Haller H, LuftFC. Angiotensin-induced inflammation and novel approaches to treatment. Adv Nephrol Necker Hosp. 2001;31:89–103.
- Takaoka M, Ohkita M, Matsumura Y. Pathophysiological role of proteasome-dependent proteolytic pathway in endothelin-1-related cardiovascular diseases. Curr Vasc Pharmacol. 2003;1:19–26.
- Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277:R922–R929.
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203.
- Han SL, Yu LX, Ma JJ, . Effect of anti-tumor necrosis factor-alpha monoclonal antibody in alleviating renal ischemia-reperfusion injury. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:332–334.
- Kim YK, Yoo JH, Woo JS, Jung JS, Kim BS, Kim SY. Effect of pentoxifylline on ischemic acute renal failure in rabbits. Ren Fail. 2001;23:757–772.
- Danis VA, Franic GM, Rathjen DA, Laurent RM, Brooks PM. Circulating cytokine levels in patients with rheumatoid arthritis: Results of a double blind trial with sulphasalazine. Ann Rheum Dis. 1992;51:946–950.
- Bissonnette EY, Enciso JA, Befus AD. Inhibitory effects of sulfasalazine and its metabolites on histamine release and TNF-alpha production by mast cells. J Immunol. 1996;156:218–223.