Abstract
The pathophysiological modifications underlying chronic renal failure seems to be dependent on the insufficiency degree, which will determine the moment to start therapy. As there is yet limited information about animal models of moderate chronic renal failure, we intended to perform a complete characterization of the hematological and cardio-renal alterations induced by partial nephrectomy. Blood samples from control and chronic renal failure rats were collected at 0, 3, 9, and 15 weeks in order to evaluate renal function, hematological parameters, iron metabolism, blood lipids, peripheral sympathetic nervous system, and inflammatory and redox status markers. BP, tissues trophy indexes, and kidney histomorphology were also assessed. Our data are consistent with a sustained moderate degree of chronic renal failure with a quickly compensated modest anaemia, though presenting iron metabolism disturbances. Despite the reasonable degree of functionality of the remnant kidney, as suggested by the anaemia correction and by the kidney hypertrophy and moderate lesions, several important cardiovascular modifications were developed. Our model presented hypertension, dyslipidemia, erythropoietic disturbances, sympathetic activation, and oxidative stress. This model might be a good tool to study the cellular/molecular mechanisms underlying moderate stages of chronic renal failure and to evaluate the therapeutic efficacy for prevention and treatment/correction of cardio-renal anaemia syndromes and complications in early stages.
INTRODUCTION
Chronic kidney disease (CKD) worsens spontaneously and progressively as the number of functioning nephrons decreases below a certain threshold. In humans, CKD has been associated with a large number of alterations, namely anaemia, hypertension, inflammation, iron metabolism disturbance, white blood cell activation, oxidative stress and sympathetic over-activation.Citation[1–6] However, the pathophysiological alterations seems to be dependent on the renal failure degree, which will also determine the moment to initiate hemodialysis and recombinant erythropoietin (rhEPO) therapies, for correction of anaemia as well as its success. Therefore, cardiovascular events, renal failure, and premature death can be better prevented or delayed if earlier identified and treated for CKD.Citation[6]
Animal models of CRF, achieved by a reduction in nephron number, create the possibility of testing in vivo the mechanisms associated to renal dysfunction, and might be essential to study the complications associated with CRF as well as the efficacy of therapeutics to prevent or delay adverse effects. The most commonly used techniques include surgical resection of the tissue (partial nephrectomy) and infarction,Citation[7] but there is yet limited information about its complete characterization, namely for moderate CRF stages, which will restrict the research on the molecular/cellular mechanism underlying the cardio-renal-anaemia syndrome, as well as its therapeutics. There is some evidence that the infarction model in rats presents a significant increase in proteinuria, hypertension and glomerulosclerosis when compared with the model of simple tissue excision (with equivalent renal mass reduction).Citation[8] This suggests that partial nephrectomy can provide a better model of renal failure in rat.Citation[7] Furthermore, there is some evidence of a good inter-individual variability of uremia in this model of CRF. However, it is difficult to standardize it, due to the lack of consistency of nephron mass reduction and, thus, to reach the desired degree of uremia.
The literature describes some features of distinct CRF models in the ratCitation[7–12]; however, they have been mainly focused on parameters related to renal function and to basic hematological studies, and even less is known about moderate renal failure models. Therefore, without a consistent characterization and of a further comparison with the human pathophysiology of moderate CKD, the research on the molecular and cellular mechanisms underlying the cardio-renal-anaemia syndrome and its therapeutics will remain limited.
The aim of our study was to perform a larger characterization of this model by studying both hematological and cardio-renal alterations in a rat model of moderate CRF induced by partial (¾) nephrectomy during a follow-up period of 15 weeks, focusing on sympathetic activity, inflammation, and oxidative stress.
MATERIALS AND METHODS
Animals and Experimental Protocol
Male Wistar rats (Charles River Lab., Inc., Barcelona, Spain) weighing ± 275 g were maintained in an air-conditioned room, subjected to 12 h dark/light cycles and given standard rat chow (IPM-R20, Letica, Barcelona, Spain) and free access to tap water. Animal experiments were conducted according to the European Communities Council Directives on Animal Care.
The rats were divided into two groups (seven rats each): a control group, and a group with surgical CRF induced by a two-stage (3/4) nephrectomy. First, about half of the left kidney was removed by left flank incision; one week later, the right kidney was removed through identical procedure. Control sham-operated rats were not required based on our preliminary studies that demonstrated a similar pattern for hematological and renal parameters with the non-operated controls after nine weeks. All of the animals have completed the 15-week protocol. Body weight (BW) was monitored during the study and blood pressure (BP) and heart rate (HR) measures using a tail-cuff sphygmomanometer LE 5001 (Letica, Barcelona, Spain).
Sample Collection and Preparation
Blood
At the beginning of the experiments and at 3, 9, and 15 weeks after the surgical partial nephrectomy, the rats were subjected to intraperitoneal anesthesia with a 2 mg/kg BW of a 2:1 (v:v) 50 mg/mL ketamine (Ketalar®, Parke-Davis, Lab., Pfizer Lda, Seixal, Portugal) solution in 2.5% chlorpromazine (Largactil®, Rhône-Poulenc Rorer, Lab. Vitória, Amadora, Portugal). Blood samples were immediately collected by venipuncture from the jugular vein into syringes without anticoagulant (for serum samples collection) or with the appropriate anticoagulant: EDTA, heparin, or a solution of acid citrate-dextrose (ACD). Blood was centrifuged (160 g for 10 min. at 20ºC) to obtain the PRP, which was then centrifuged (730 g for 10 min. at 20ºC) to obtain the platelet pellet and the PPP. In order to maintain a normal volemia, thus ensuring that results were not changed by the amount of blood collected, the following parameters were analyzed only at the final time (15 weeks) in a 10 ml blood sample collected from rats under the anesthesia listed previously: circulating catecholamines contents and inflammatory and redox status markers. In the earlier stages, only 2 ml of blood were collected.
Tissues
The rats were sacrificed by cervical dislocation, and the heart, adrenals, kidneys, and liver were immediately removed, placed in ice-cold Krebs' buffer, and carefully cleaned of adherent fat and connective tissue. The BW, the whole heart weight (HW), the left ventricle weight (LVW), the two adrenals weight (AW), and the kidney and liver weights were measured in all the rats under study in order to be used as trophy indexes. Kidney histomorphology was analyzed through hematoxylin-eosin (H&E) staining.
Assays
Renal and Liver Function
Serum creatinine, urea, and uric acid concentrations were used as renal function indexes, and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were assessed for liver evaluation, through automatic validated methods and equipments (Hitachi 717 analyser).
Blood Lipid
Serum total cholesterol (Total-c), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) and triglycerides (TGs) were analyzed on a Hitachi 717 analyser (Roche Diagnostics Inc., Massachuasetts, USA) using standard methods. LDL-c/HDL-c and Total-c/HDL-c were used as atherogenic and cardiovascular risk indexes.
Iron Metabolism
Serum iron concentration was determined using a colorimetric method (Iron, Randox Laboratories Ltd., North Ireland, UK), whereas serum ferritin and transferrin were measured by immunoturbidimetry (Laboratories Ltd., North Ireland, UK).
Hematological Data
Red blood cell (RBC) count, hematocrit, hemoglobin (Hb) concentration, hematological indices [mean cell volume (MCV), mean cell Hb (MCH) and mean cell Hb concentration (MCHC)], red cell distribution width (RDW), reticulocyte count, immature reticulocyte fraction (IRF), platelets count, platelets indices [mean platelet volume (MPV), platelet distribution width (PDW) and plaquetocrit (PCT)], and total and differential white blood cell (WBC) counts were assessed in whole blood EDTA by using an automatic Coulter Counter® (Beckman Coulter Inc., California, USA). Serum erythropoietin was measured by using an immunoassay kit (R&D Systems, Minneapolis, Minnesota, USA). Results were expressed in pg/mL.
Catecholamine Assay
Norepinephrine (NE) and epinephrine (E) concentrations in plasma, platelet, adrenals, and kidney were evaluated by high-performance liquid chromatography with electrochemical detection (HPLC-ED), according to previously described,Citation[13] using appropriate standards (Sigma Chemical Co. (St. Louis, Missouri, USA) and software (Gilson 710). Concentrations were expressed in ng/mL for plasma and platelets, μg/g wet tissue for adrenals, and ng/g for kidney.
Inflammatory Markers
Serum levels of interleukin 2 (IL-2), IL-1β, transforming growth factor β1 (TGF-β1), and tumor necrosis factor α (TNF-α) were measured by ultrasensitive Quantikine® ELISA kits (R&D Systems, Minneapolis, Minnesota, USA). Serum C-reactive protein (CRP) was determined by using an ELISA kit from Helica Biosystems, Inc. (Fullerton, California, USA). All assays were performed in duplicate.
Redox Status
The thiobarbituric acid reactive-species (TBARs) assay was used to assess serum products of lipid peroxidation, namely malondialdehyde (MDA).Citation[14] Ferric reducing antioxidant potential (FRAP) assay was used to estimate serum total antioxidant status (TAS).Citation[15] Serum 3-nitrotyrosine (3-NT), which is an index of peroxynitrite formation, was measured through an immunoassay (HyCult biotechnology b.v., Uden, Netherlands).
Statistical Analysis
For statistical analysis, we used the Statview 4.53 software from Abacus Concepts Inc. (Berkeley, California, USA). Results are presented as means ± standard error of means (SEM). Comparisons between groups and between different times of evaluation were performed using one-way ANOVA and Fisher's test. Significance was accepted at p less than 0.05.
RESULTS
Biochemical and Hematological Data
In and , we present the biochemical and hematological changes for CRF and control rats, before starting experiments and along the experimental period (at 3, 9, and 15 weeks after partial nephrectomy). The results were analyzed in order to study the alterations associated with a moderate CRF during 15 weeks of follow-up, as compared to the control group. In CRF rats, three weeks after the partial (¾) nephrectomy, a statistically significant increase in serum urea and creatinine concentrations were found. This increase in renal function markers remained high along the following 12 weeks (see ). Concerning liver function markers, a statistically significant increase was found in renal failure rats for AST and ALT, particularly for the former at 9 weeks of the experimental period; at the end of experiments (15 weeks), only AST activity was still significantly higher. CRF rats presented alterations in the lipidic profile, namely, a progressively increase in Total-c, TGs, and HDL-c (see ). Iron status evaluation revealed in CRF rats a progressively increase in ferritin serum levels, which reached statistical significance in the last evaluation. On the contrary, transferrin serum levels were significantly lower three weeks after surgical procedure, and remained lower during the following 12 weeks of follow-up (see ).
Table 1 Biochemical changes in a rat model of moderate CRF during a follow-up period of 15 weeks
Table 2 Hematological changes in a rat model of moderate CRF during a follow-up period of 15 weeks
In relation to hematological data, three weeks after nephrectomy, the CRF animals showed a statistically significant decrease for RBC count, Hb, and hematocrit, alongside a significantly increase in RDW and in platelet count (see ). These parameters were already similar to those of the control at 9 weeks after surgical intervention and remained stable until the end of the follow-up period. A trend to lower serum EPO levels was obtained in the CRF rats (19.43±3.05 pg/mL) vs. the control (22.25 ± 1.00 pg/mL) at the final time, but not reaching statistical significance.
No statistically significant differences were found for total WBC counts; however, neutrophil and eosinophil counts increased progressively in renal failure rats. In this group, basophil counts increased three weeks after chirurgic procedure, and decreased progressively during the remaining follow-up period, and monocyte counts increased only in the last laboratorial evaluation (see ).
Blood Pressure, Heart Rate, Tissue Trophic Index, and Kidney Histomorphology
At the end of the experimental protocol, a statistically significant increase in SBP, DBP, MBP, and HR were found in CRF rats, together with increment in heart and LV ventricle, without statistically significant differences in tissue trophic indexes (see ).
Table 3 Cardio-renal characterization, inflammatory markers, and redox status profile in a rat model of moderate CRF after 15 weeks of experimental study
The kidney morphology of the renal failure rats was distinct from that of the controls: the glomerular capillary tufts of the renal failure rats were hypercellular, with an increment of the glomerular volume; the Bowman space was also higher; the interstitial region was lower due to tubular atrophy, together with expansion of proximal convoluted tubules (data not shown).
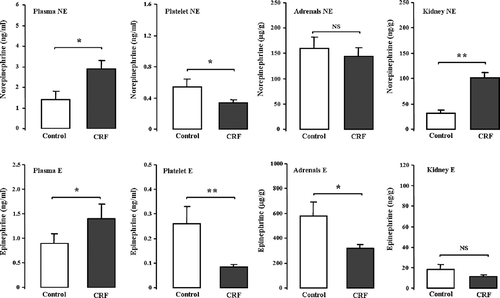
Catecholamine Measures
Concerning sympathetic activity, CRF rats presented a statistically significant increase in plasma and kidney NE, and a decrease in platelet content together with a significant reduction in platelet and adrenals E concentrations, and a concomitant increment in plasma E (see).
Figure 1. Norepinephrine (NE) and epinephrine (E) contents in plasma, platelets, adrenals, and kidneys in a rat model of moderate CRF after an experimental period of 15 weeks. Results are mean ± SEM of n = 7 rats/group. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group. Abbreviation: NS = non-significant.
Redox Status and Inflammatory Profile
No statistically significant alterations were found between the two rats groups for MDA and TAS, but a significantly higher serum concentration of 3-NT was found. Concerning the inflammatory profile, we observed no significant differences between the two groups for CRP, IL-1β, IL-2, and TNF-α, except for TGF-β1, which was augmented in CRF rats (see ).
DISCUSSION
The CKD is a major public health problem throughout the world. The major outcomes of chronic renal failure include rapid progression, with development of anaemia and serious complications, namely cardiovascular events. Early detection of renal failure and initiation of treatment should contribute to prevent or delay some of those adverse effects.Citation[16] However, animal models of moderate CRF, which might be good tools to study the pathophysiological mechanisms underlying intermediary stages of renal disease and the efficacy of therapeutics, remain to be fully characterized.
Our results have confirmed that the surgical partial (3/4) nephrectomy produces a moderate but sustained stage of CRF. Indeed, we observed a significant (but restrained) increase in serum urea and creatinine concentrations three weeks after the surgery that remained almost constant during the following period (still significantly higher than controls). Furthermore, the results observed for RBC and reticulocyte counts were similar to those observed for serum urea and creatinine along the follow-up period, further supporting that a moderate stage of renal failure was generated. RBC count was significantly lower three weeks after nephrectomy, consistent with the development of anaemia secondary to renal mass reduction; moreover, the number of reticulocytes as well as the percentage of immature reticulocytes did not increase, which suggests a failure of the erythropoietic response mechanisms due to a putative insufficient erythropoietin production associated to the reduction in renal tissue. However, the anaemia was notoriously transitory, as both RBC and reticulocyte values returned to normal values in the following evaluation points (9 and 15 weeks of study), suggesting that our model is a moderate (but yet functional) CRF. Furthermore, at the final time, the serum EPO levels were almost unchanged between the control and the CRF rats. This pattern is also confirmed by the low-grade histomorphological changes (lesions) found in the kidneys of CRF rats. There was also a trend to increased kidney weight (hypertrophy), consistent with a compensated renal insufficiency. Similar hypertrophy was obtained in other models of CRF induction, such as the 5/6 nephrectomized rats.Citation[9–12] Furthermore, the CRF rats presented a notable increment in serum TGF-β1 levels, which is in agreement with the mass recovering of the remnant kidney, most probably due to mechanisms of cell growth and differentiation, hypertrophy, and apoptosis, which have been implicated in the chronic kidney disease pathophysiology.Citation[17]
Iron-restricted erythropoiesis is a common clinical condition in patients with CKD. Several causes were proposed to underlie this situation, such as a functional iron deficiency, inadequate dietary iron intake, blood loss during the hemodialysis processes or from gastrointestinal tract (bleeding), inadequate intestinal iron absorption, and inhibition of iron mobilization from macrophages.Citation[12–16,Citation18] In our study, CRF rats presented serum iron values similar to those of the control, and no significant changes were observed along the experiments; however, a trend toward higher values of ferritin were observed along the experiments and reached a significantly higher value at the end (15 weeks). These changes in ferritin were accompanied by a significant reduction in transferrin (observed at 3 weeks and afterward), suggesting an inhibition in iron traffic, from macrophages to erythroid cells, leading to the progressive increase in iron storage, as shown by the progressive increase in ferritin observed throughout the experiments. This suggests that our moderate model of CRF could also be used as a model of functional iron deficiency. Indeed, a functional iron deficiency has been reported in CKD patient under hemodialysis, namely, in moderate stages.Citation[18,Citation19]
Concerning the other biochemical parameters, our model demonstrated important changes in liver function markers, with AST and ALT being significantly higher at 9 weeks and at the end of experiments in CRF rats, accompanied by a trend to increased liver weight. We might hypothesize that renal dysfunction leads to a progressive deterioration of liver function, but the exact interacting mechanism(s) between the kidney and the liver deserve further clarification. Furthermore, the increase in AST without changes in ALT, which occurred at the end of experiments (15 weeks), might reflect other non-hepatic dysfunctions, namely on the cardio-renal axis, which is supported by other data from our work, such as by the hypertrophic heart and kidneys.
Liver metabolism dysfunction could also play a role in the lipid profile changes encountered in the CRF rats: increased values of total-cholesterol, HDL-c, and TGs without differences on LDL-c content, which might be explained, at least in part, by the lack in cholesteryl ester transfer protein (CETP), a feature of the rats.Citation[20] Lipid abnormalities are found in CKD humans, and the prevalence of hyperlipidemia and metabolic syndrome is higher than in the general population.Citation[21,Citation22] However, the risk of cardiovascular disease in CKD patients varies depending on the type of lipid abnormalities, the cause of renal disease, and the degree of reduction in glomerular filtration rate (GFR).Citation[23] Our rat model reproduces the lipidic changes encountered in CKD patients.Citation[20–23]
Besides the anaemia secondary to renal failure, CKD patients usually develop cardiac failure that further aggravates renal disease.Citation[24,Citation25] This triad of dysfunctions, already known as cardio-renal anaemia syndrome, is responsible for the serious complications encountered in those patients. Our study intended to clarify the degree of cardio-renal complications associated with this model of moderate renal failure. Hypertension is a well-established cause, a common complication, and an important risk factor for the progression of the cardiovascular complications and the mortality of patients with CRF.Citation[6] Our results confirmed that condition, with increased SBP and DBP, together with tachycardia and heart and left ventricle hypertrophy, which should be due to additional effort to compensate the renal function deterioration. The pathophysiology of this hypertension is multifactorial.Citation[26] Several authors conclude that CRF also activates (through the renin-angiotensin system) the sympathetic nervous system (SNS).Citation[5,Citation16] The kidneys, strategically positioned, have dense sensory and efferent sympathetic innervations, and can be the origin as well as the target of overactivity of the SNS, which has been convincingly shown in CRF animal models.Citation[27] Several studies showed that partially nephrectomized rats developed a rapid increase of BP within a week after renal ablation, while totally nephrectomized rats, in which afferent sensory signals were removed, did not develop hypertension. This suggests that afferent signals from the disease kidneys are transmitted to the vasomotor control center in the brain, thereby contributing to the increased BP.Citation[5,Citation27] In our model of moderate renal failure, plasma NE and E contents were increased, which might be caused by adrenal and platelet release, due to sympathetic system over-activation and platelet hyper-reactivity, respectively, both contributing to cardiovascular and thromboembolic complications as well as atherosclerosis found in CKD patients.Citation[28,Citation29]
Other factors have been studied to clarify the causes of this hypertension, including high levels of vasoconstrictors, oxidative stress or nitric oxide (NO) reduction.Citation[6,Citation26–31] In CRF patients, oxidative stress and inflammation could play a crucial role in the pathogenesis of the atherosclerosis, malnutrition, and anaemia. In our model of moderate renal failure, the redox state seems to be unaltered (MDA/TAS ratio was unchanged), which might be due to a proper compensation of ROS formation by antioxidants. However, there was an increased serum 3-NT value, which is a marker of peroxynitrite generation and thus might reflect an increased superoxide formation and a reduced NO availability, as this dangerous oxidant is formed by the combination of both. In this model, inflammation seem to be yet less relevant as, except for an increment of TGF-β1, all of the other markers were unchanged. The increment in ferritin, an acute phase protein, might also be viewed as part of an inflammatory state. However, the involvement of inflammation on this model of moderate CRF should be further confirmed, namely, by studies on the hypertrophic remnant kidney.
In conclusion, this model is consistent with a moderate degree of renal failure (sustained during the follow-up period) with a modest and quickly compensated anaemia, though presenting a disturbance in iron metabolism. Despite the reasonable degree of functionality of the remnant kidney (1/4 of total mass), as suggested by the correction of anaemia as well as by the kidney hypertrophy and the moderate structural modifications (lesions) observed, several important cardiovascular modifications were developed. Therefore, our model presented hypertension, dyslipidemia, erythropoietic disturbances, sympathetic activation, and oxidative stress. This model might be a good tool to study the cellular/molecular pathophysiological mechanisms underlying moderate stages of chronic renal failure, as occurs in humans, and, even more relevant, to evaluate the efficacy of therapeutics for prevention, treatment, or correction of cardio-renal anaemia syndromes and complications in early stages.
ACKNOWLEDGMENTS
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Parmar MS. Chronic renal disease. BMJ. 2002;325:85–90.
- Kerr PG. Renal anemia: Recent developments, innovative approaches and future directions for improved management. Nephrology (Carlton). 2006;11:542–548.
- Stenvinkel P. Anaemia and inflammation: What are the implications for the nephrologists? Nephrol Dial Transplant. 2003;18:viii17–viii22.
- Costa E, Pereira BJ, Rocha-Pereira P, . Role of prohepcidin, inflammatory markers and iron status in resistance to rhEPO therapy in hemodialysis patients. Am J Nephrol. 2008;28:677–683.
- Koomans HA, Blankestijn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure: A wake-up call. J Am Soc Nephrol. 2004;15:524–537.
- Bakris GL. Protecting renal function in the hypertensive patient: Clinical guidelines. Am J Hypertens. 2005;18:112S–119S.
- Liu ZC, Chow KM, Chang TM. Evaluation of two protocols of uremic rat model: Partial nephrectomy and infarction. Ren Fail. 2003;25:935–943.
- Ofstad J, Horvei G, Kvam FI, . Glomerular hemodynamics in progressive renal disease. Kidney Int. 1992;36:S8–S14.
- Fine L. The biology of renal hypertrophy. Kidney Int. 1986;29:619–634.
- Gretz N, Waldherr R, Strauch M. The remnant kidney model. In: Gretz N, Strauch M ( eds.). Experimental and genetic rat models of chronic renal failure. Basel: Karger; 1993:1–28.
- Hostetter TH, Olson JL, Rennke HG, . Hyperfiltration in remnants nephrons: A potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12:1315–1325.
- Toblli JE, Cao G, Rivas C, Kulaksiz H. Heart and iron deficiency anaemia in rats with renal insufficiency: The role of hepcidin. Nephrology (Carlton). 2008;13:636–645.
- Reis F, Rocha L, Ponte L, . Effect of preventive and regressive isosorbide 5-mononitrate treatment on catecholamine levels in plasma, platelets, adrenals, left ventricle and aorta in cyclosporin A-induced hypertensive rats. Life Sci. 2005;77:2514–2528.
- Estepa V, Ródenas S, Martín MC. Optimización de un método para la determinación de la peroxidación lipídica en suero humano. Anal Real Acad Farm. 2001;67:1–17.
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–76.
- Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: Renoprotective benefits of renin-angiotensin system inhibition. Ann Intern Med. 2002;136:604–615.
- Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T. TGF-beta signal transduction in chronic kidney disease. Front. Biosci. 2009;14: 2448–2465.
- Hörl WH. Iron therapy for renal anemia: How much needed, how much harmful? Pediatr Nephrol. 2007;22:480–489.
- Brugnara C. Iron deficiency and erythropoiesis: New diagnostic approaches. Clin Chem. 2003;49:1573–1578.
- Oschry Y, Eisenberg S. Rat plasma lipoproteins: Re-evaluation of a lipoprotein system in an animal devoid of cholesteryl ester transfer activity. J Lipid Res. 1982;23:1099–1106.
- Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: An approach to pathogenesis and treatment. Am J Nephrol. 2008;28:958–973.
- Johnson DW, Armstrong K, Campbell SB, . Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton). 2007;12:391–398.
- Chan CM. Hyperlipidemia in chronic renal disease. Ann Acad Med Singapore. 2005;35:31–35.
- Silverberg D, Wexler D, Blum M, . The cardio-renal anaemia syndrome: Does it exist? Nephrol Dial Transplant. 2003;18:viii7–viii12.
- Wexler D, Silverberg D, Blum M, . Anaemia as a contributor to morbidity and mortality in congestive heart failure. Nephrol Dial Transplant. 2005;20:vii11–vii15.
- Ritz E, Koomans HA. New insights into mechanisms of blood pressure regulation in patients with uremia. Nephrol Dial Transplant. 1996;11:52–59.
- Campese VM. Neurogenic factors and hypertension in chronic renal failure. J Nephrol. 1997;10:184–187.
- Malyszko J, Malyszko JS, Pawlak D, . Homeostasis, platelet function and serotonin in acute and chronic renal failure. Thromb Res. 1996;83:351–361.
- Hojs R, Hojs-Fabjan T, Balon BP. Atherosclerosis in patients with end-stage renal failure prior to initiation of hemodialysis. Ren Fail. 2003;25:247–254.
- Martín-Mateo MC, Sánchez-Portugal M, Iglesias S, de Paula A, Bustamante J. Oxidative stress in chronic renal failure. Ren Fail. 1999;21:155–167.
- Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25: 311–317.