Abstract
Background. Hemodialysis (HD) patients are susceptible to atypical tuberculosis (TB), especially among patients presenting with fever of unknown origin (FUO), because of their impaired cellular immunity. Diagnostic trials of anti-TB drugs are therefore recommended in some TB endemic countries, including Japan, though clinical evidence for this therapy is scarce. Methods. We prospectively collected data for incident cases of clinical FUO for two years in 78 of 169 dialysis facilities in Aichi prefecture, located in central Japan. Clinical FUO was defined as sustained fever without any localizing signs and no infiltration on chest x-rays after a one-week antibiotic trial. The baseline characteristics, subsequent body temperatures on the days of HD therapy, and names of antibiotics including anti-TB drugs with the durations of medication were reported until fever alleviation or fever sustainment for over eight weeks. Results. We identified 15 newly developed clinical FUO patients among 8,125 HD patients. The incidence rate was estimated to be 92 (95% CI, 26–158) per 100,000 person-years. This corresponds to 244 cases per year among 264,473 HD patients in Japan. Anti-TB drugs were secondarily prescribed in 8 of 15 clinical FUO patients (53%). No improved fever alleviation was observed when anti-TB drugs were secondarily prescribed compared with cases in which other antibiotics were preferred. Conclusion. We investigated the incidence of FUO in HD patients and found that the rate was not very high, whereas anti-TB drugs were frequently used for FUO cases. The efficacy of this diagnostic therapy should be elucidated in large-scale studies.
INTRODUCTION
Cellular immunity, which mediates host resistance to mycobacterium tuberculosis (TB), is impaired in patients with end-stage renal disease (ESRD).Citation[1] Accordingly, TB occurs more frequently in hemodialysis (HD) patients than in the general population,Citation[2] and its appearance is often atypical.Citation[3,Citation4] Extrapulmonary TB comprises more than half of all TB cases,Citation[2,Citation5] and fever of unknown origin (FUO) is the most common manifestation in HD patients.Citation[5] It is known that TB leading to FUO is either extrapulmonary tuberculosis without clear localizing signs or miliary tuberculosis without any chest X-ray findings.Citation[6] These observations suggest that ESRD patients are particularly susceptible to atypical TB presenting with FUO.
So-called diagnostic anti-TB trials are recommended for ESRD patients with FUO in some TB endemic countries,Citation[2,Citation7–9] including Japan,Citation[10,Citation11] and TB is diagnosed according to the responses to these trials.Citation[8,Citation12–15] However, clinical evidence for this therapy is scarce.
Classic FUO is defined as FUO lasting for more than three weeks,Citation[16] but empiric antibiotic therapy is often initiated early under the tentative diagnosis of bacterial infection when the clinical presentation is severe.Citation[17,Citation18] In the present study, we surveyed clinical FUO, defined as fever without clear localizing signs that was sustained even after a one-week antibiotic trial. We conducted a prospective observational study to estimate the incidence of clinical FUO in HD patients and the frequency of anti-TB drug use with its effectiveness.
PATIENTS AND METHODS
Study Population
In Japan, maintenance HD is usually provided to ESRD patients three times a week in a medical institute. We prospectively established a cohort of maintenance HD patients in Aichi prefecture, central Japan. All of the 169 dialysis facilities (hospitals or clinics) in Aichi prefecture were invited to participate in this study in June 2006, and 78 of them applied for enrollment in the study and were registered as the study sites. All of their maintenance HD patients were enrolled unless they refused after the announcement of this study. The total number of HD patients at each study site was reported at the time of enrollment (September 2006), and this was considered to be the study population at risk throughout the study. The total number of patients under HD therapy in Aichi prefecture and throughout Japan at the end of 2006, as well as the characteristics of patients under HD therapy in Japan, was obtained from the Annual Statistical Report by the Japanese Society for Dialysis Therapy.Citation[19] The study was approved by the Ethics Committee of Nagoya University Graduate School of Medicine (approval number, 391).
Definition of Clinical FUO
We defined clinical FUO as sustained fever (1) above 37.5°C for at least three HD visits, (2) after a one-week antibiotic trial for suspected bacterial infection, and (3) without any clear localizing signs and no infiltration shadows on a chest x-ray. Fever alleviation was defined as a body temperature reduction to less than 37.0°C for four HD visits. The antibiotics used incorporated all bactericidal and bacteriostatic agents, including quinolones and anti-TB drugs. Patients with rapid test-positive influenza, common cold with runny nose and sore throat, known malignant diseases or connective tissue diseases, and acute renal failure were excluded from the study.
Data Collection
The physicians at the study sites completed a case report form for patients who newly developed clinical FUO. In this form, the date of fever onset, subsequent body temperatures on the days of HD therapy, names of antibiotics including anti-TB drugs with the durations of medication, underlying disease for renal failure, duration of HD therapy, presence of comorbid diabetes mellitus, use of steroid hormones or immunosuppressants, and past use of anti-TB drugs were documented. Findings from physical examinations (e.g., neck stiffness, throat reddishness, abdominal tenderness, and costovertebral angle tenderness) and laboratory tests (e.g., white blood cell [WBC] counts, serum C-reactive protein [CRP] levels, and pleural effusion or infiltration suggesting old TB on a chest x-ray) were also reported.
The study end point was either fever alleviation or fever sustainment for more than eight weeks. The final diagnosis of each patient was made by their physician at the end of the follow-up based on laboratory test results or clinical features. TB was presumed by positive responses to anti-TB trials or by assay data for serum interferon-gamma.Citation[20] When a patient with clinical FUO was referred to another hospital, the case report form was also transferred.
Statistical Analysis
The incidence rate and 95% confidence interval (CI) of clinical FUO under HD therapy were estimated. The background characteristics were compared according to the outcome (fever alleviation or fever sustainment) by Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for numerical variables. Kaplan-Meier survival curves for fever alleviation after the second prescription were drawn according to the therapeutic regimens (anti-TB drugs or other antibiotics). The analyses were carried out using the software STATA ver. 9 (StataCorp, Texas, USA), and values of p < 0.05 were regarded as statistically significant.
RESULTS
A total of 8,125 patients were under HD therapy at the study sites at the beginning of the study, accounting for 58% of all HD patients in Aichi prefecture. The amount of study person-time was 16,250 person-years. Among these patients, 15 newly developed clinical FUO. The incidence rate of clinical FUO was estimated to be 92 (95% CI, 26–158) per 100,000 person-years, corresponding to 13 cases among 14,038 HD patients in Aichi prefecture and 244 cases among 264,473 HD patients throughout Japan.
The baseline characteristics of the patients with clinical FUO are shown in . Female patients were more likely to exhibit sustained fever. A shorter HD duration tended to be associated with fever sustainment. WBC counts and serum CRP levels at inclusion were higher in patients with subsequent fever alleviation. Pleural effusion on a chest x-ray was more common among patients with sustained fever. However, none of these findings reached statistical significance. Overall, the characteristics of the patients with clinical FUO did not differ markedly from those of HD patients in Japan (mean age, 64 vs. 65 years; females, 47% vs. 39%; diabetic nephropathy, 33% vs. 33%; duration of HD under 5 years, 47% vs. 50%).
Table 1 Baseline characteristics of patients with clinical FUO according to their fever alleviation
shows the final diagnoses of the patients with clinical FUO. Overall, one-third of the patients were diagnosed as having definite or probable TB, and one-half of the patients were diagnosed as having probable bacterial infection other than TB. The proportion of presumed bacterial infections was somewhat higher in the alleviated fever group (60%) than in the sustained fever group (20%). The proportion of fever alleviation did not differ significantly between patients with definite/probable TB and patients with probable bacterial infections (60% vs. 86%, p = 0.523).
Table 2 Diagnoses of clinical FUO at the end of follow-up according to fever alleviation
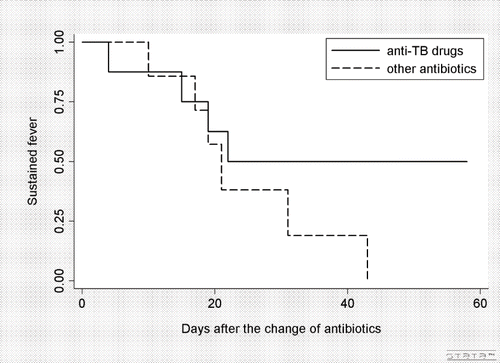
The first-choice antibiotics were not anti-TB drugs for any of the 15 clinical FUO patients. Anti-TB drugs were secondarily prescribed in 8 of the 15 patients (53%). The median durations from fever onset to the second antibiotic prescription were 22 days (interquartile range, 9 to 31) for anti-TB drug users and 9 days (7 to 11) for the other patients. The data for fever alleviation after the second prescription are shown in . Until day 20, the curves were almost identical between the anti-TB drug group and the other antibiotics group. After day 20, no fever alleviation was observed among the patients treated with anti-TB drugs.
DISCUSSION
To the best of our knowledge, this is the first study to reveal the incidence of FUO in HD patients. The estimated incidence rate was 92 per 100,000 person-years, with the total number of such patients being 244 in Japan. Although fully comparable data are not available, this rate does not seem to be very high, based on reference to the only report on FUO incidence (2.9% among patients admitted to hospitalCitation[21]). Because Japan is still an endemic area of TB (incidence rate, 20 per 100,000 person-yearsCitation[22]) and the proportion of patients under HD therapy is high (1 in 500 peopleCitation[19]), the estimated frequency of HD patients with FUO in the present study may be greater than those in Western countries.
The duration of HD therapy was relatively short in patients with sustained fever, consistent with a previous report from Taiwan.Citation[2] This would be explained by the profoundly depressed immune defense of the patients soon after the induction of dialysis.Citation[1] The WBC counts and serum CRP levels at inclusion were greater in patients with alleviated fever, but similar findings and their mechanisms have not previously been reported. We suppose that these differences may be caused by the eradication of pathogens by the dramatic inflammation process.
We found no improved fever alleviation when anti-TB drugs were secondarily prescribed compared with cases in which other antibiotics were preferred. Anti-TB drugs may be given to patients with severe illness, resulting in the cancellation of the treatment effects. After a longer period of fever, however, anti-TB drugs could have solved the fever in one-half of the clinical FUO patients. Therefore, we suggest that clinicians should consider the possibility of anti-TB trials in the setting of clinical FUO without any positive test findings for TB.
We did not use the criteria of classic FUO. Physicians often worry about delayed administration of antibiotic therapies for immune-deficient HD patients, and early antibiotic trials without any microbial proof are quite common in clinical settings. Consequently, the terms acute and prolonged FUO have recently been proposed for fever without immediately apparent etiology.Citation[23] Our clinical FUO criteria would also be of great help in clinical practice.
A limitation of this study is the smaller number of incident cases of clinical FUO than expected. Therefore, we could not adjust for potential confounders by multivariate analysis. To minimize missing cases, we sent newsletters to all the study physicians twice a year to remind them to report new FUO cases. We also presented the outline and progress of this study at some regional conferences of nephrologists.
We confirmed the frequent use of anti-TB drugs for FUO cases, as recommended in several reports.Citation[2,Citation7–11] However, fever alleviation did not differ much between anti-TB drug users and non-users. Further studies accumulating data for FUO cases are warranted to clarify the efficacy of anti-TB trials.
ACKNOWLEDGMENTS
We appreciate the special support of the Aichi Prefectural Association of Dialysis Physicians. This study was partly supported by a Grant-in-Aid from the Aichi Kidney Foundation.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533.
- Chou KJ, Fang HC, Bai KJ, Hwang SJ, Yang WC, Chung HM. Tuberculosis in maintenance dialysis patients. Nephron. 2001;88:138–143.
- Vachharajani T, Abreo K, Phadke A, Oza U, Kirpalani A. Diagnosis and treatment of tuberculosis in hemodialysis and renal transplant patients. Am J Nephrol. 2000;20:273–277.
- Chuang FR, Lee CH, Wang IK, Chen JB, Wu MS. Extrapulmonary tuberculosis in chronic hemodialysis patients. Ren Fail. 2003;25:739–746.
- Fang HC, Lee PT, Chen CL, Wu MJ, Chou KJ, Chung HM. Tuberculosis in patients with end-stage renal disease. Int J Tuberc Lung Dis. 2004;8:92–97.
- Liu KS, Sheng WH, Chen YC, Chang SC, Hsieh WC. Fever of unknown origin: A retrospective study of 78 adult patients in Taiwan. J Microbiol Immunol Infect. 2003;36:243–247.
- Yuan FH, Guang LX, Zhao SJ. Clinical comparisons of 1,498 chronic renal failure patients with and without tuberculosis. Ren Fail. 2005;27:149–153.
- Erkoc R, Dogan E, Sayarlioglu H, Tuberculosis in dialysis patients, single centre experience from an endemic area. Int J Clin Pract. 2004;58:1115–1117.
- Bofinger JJ, Schlossberg D. Fever of unknown origin caused by tuberculosis. Infect Dis Clin North Am. 2007;21:947–962.
- Inamoto H. Tuberculosis in dialysis patients. Journal of Japanese Society for Dialysis Therapy. 1987;20:165–176 [in Japanese].
- Furukawa A, Hashine K, Miyamoto T, Study of fever of unknown origin in patients on maintenance dialysis. Journal of Japanese Society for Dialysis Therapy. 1991;24:163–166 [in Japanese].
- Collazos J, Guerra E, Mayo J, Martínez E. Tuberculosis as a cause of recurrent fever of unknown origin. J Infect. 2000;41:269–272.
- Malik GH, Al-Harbi AS, Al-Mohaya S, Eleven years of experience with dialysis associated tuberculosis. Clin Nephrol. 2002;58:356–362.
- Cengiz K. Increased incidence of tuberculosis in patients undergoing hemodialysis. Nephron. 1996;73:421–424.
- Onal IK, Cankurtaran M, Cakar M, Fever of unknown origin: what is remarkable in the elderly in a developing country?. J Infect. 2006;52:399–404.
- Durack DT, Street AC. Fever of unknown origin—reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51.
- Cunha BA. Fever of unknown origin: Clinical overview of classic and current concepts. Infect Dis Clin North Am. 2007;21:867–915.
- Bryan CS, Ahuja D. Fever of unknown origin: Is there a role for empiric therapy?. Infect Dis Clin North Am. 2007;21:1213–1220.
- The Japanese Society for Dialysis Therapy. The Annual Statistical Report 2006. Available at. http://docs.jsdt.or.jp/overview/index2007.html (Nov. 2008).
- Chin C, Lee SS, Chen YS, Mycobacteriosis in patients with fever of unknown origin. J Microbiol Immunol Infect. 2003;36:248–253.
- Iikuni Y, Okada J, Kondo H, Kashiwazaki S. Current fever of unknown origin 1982–1992. Intern Med. 1994;33:67–73.
- Research Institute of Tuberculosis Japan. Statistics of TB 2006. Available at. http://www.jata.or.jp/rit/re/epro1_ top.htm (November 2008).
- Bryan CS. Fever of unknown origin: the evolving definition. Arch Intern Med. 2003;163:1003–1004.
APPENDIX
The investigators who were involved in the FUO-HD Study (and their affiliations) are as follows: Shizunori Ichida (Nagoya First Red Cross Hospital); Daijyo Inaguma (Tosei General Hospital); Yasumitsu Mori (Ama Kyoritsu Clinic); Kimihiro Takayama (Aoi Central Hospital); Tomohiko Naruse (Kasugai Municipal Hospital); Akira Ohno (Komaki Clinic); Takeyuki Hiramatsu (Konan Kosei Hospital); Fumi Kato and Chikao Yamasaki (Masuko Memorial Hospital); Masato Yamakawa (Minato Kyoritsu General Hospital); Atsuhiro Yoshida (Nagoya City University Graduate School of Medical Science); Hiroaki Asada (Okazaki City Hospital); Hideo Tawada (Tawada Hospital); Masato Sakuma (Aichi Medical University Hospital); Hiroshi Tamai (Anjo Kosei Hospital); Masato Tsuboi (Anjo Kyoritsu Clinic); Takuya Ueda (Aoi Clinic Nishi-Okazaki); Masamiki Miwa (Atsuta Clinic); Yoshiro Fujita and Hideaki Shimizu (Chubu Rosai Hospital); Mikito Tsuyuki (Chukyo Hospital); Tomoya Tayasu and Shinken Somiya (Clinic Tsushima); Akira Ohya (Daido Hospital); Izumi Shimozato (Daini Shimozato Clinic); Akikazu Yamamoto (Hakuyokai Hospital); Midoriko Watanabe (Handa City Hospital); Shingo Masamoto (Handa Clinic); Noboru Hirajima (Handa Higashi Clinic); Takamasa Kawade (Handa Kyoritsu Clinic); Hideo Ito (Hekikai Kyoritsu Clinic); Satoko Awata (Hekinan Clinic); Tatsuya Ohta (Itsuki Clinic); Tatsuo Kato (Johoku Clinic); Yoshihiro Ohta, Miho Sarai and Hideo Sugihara (Juzen Clinic); Hiroyuki Morita (Kaikokai Central Clinic); Masaru Kato (Kamiiida Clinic); Hisatsugu Kurata (Kamo Hospital); Shin-ichirou Iyoda (Kanayama Clinic); Takafumi Nomura (Kasugai Central Clinic); Yasunobu Shimano and Hiroshi Sakai (Kawana Hospital); Masami Ohyama (Kisogawa City Hospital); Yoshiyasu Iida (Komaki City Hospital); Kachiko Morikawa (Koujukai Rehabilitation Hospital); Yuko Kinoshita (Masuko Clinic Subaru); Yasushi Suzuki (Meijo Hospital); Yoshinari Tsuruta (Meiyo Clinic); Naoyuki Miyagawa (Miai Clinic); Saeko Morikawa (Mikawa Clinic); Masao Mizuno (Mizuno Clinic); Eiko Soga (Mizuno Clinic Mizuhiro Branch); Masaki Kobayashi (Moriyama Yuai Hospital); Hiroaki Asada (Mutsumi Medical Clinic); Kazuo Miyatani (Nagoya Higashi Clinic); Hiroki Kasuga (Nagoya Kyoritsu Hospital); Masako Sakakibara and Hiromitsu Kusafuka (Nagoya Memorial Hospital); Naoyuki Fukuda (Nagoya Sakae Clinic); Yoshihiro Tominaga and Kunio Morozumi (Nagoya Second Red Cross Hospital); Sachiyo Namiki (Namiki Hospital); Masayasu Narita (Narita Memorial Hospital); Itsuo Yokoyama (Narumi Clinic); Atsushi Nomura (Nomura Medical Clinic); Kazumi Ohno (Ohno Urological Clinic); Ryoji Sassa (Okazaki Kita Clinic); Tadashi Ogawa (Okehazama Clinic); Toshifumi Tetsuka (Owarinishi Clinic); Yoshinobu Iyoda (Saijo Hospital); Yuji Hatanaka (Sakashita Clinic); Toshiyuki Hashimoto (Satoh Hospital); Hiroshi Ogawa (Shinseikai Daiichi Hospital); Munetaka Sugiishi (Sugiishi Hospital); Midori Koide (Sugiyama Hospital); Naganari Watarai (Takasu Hospital); Hisae Tawada (Tawada Clinic); Toyohiko Watanabe (Terada Clinic); Haruo Niwa (Toei Hospital); Tsunehisa Sakurai (Togo Haruki Clinic); Makoto Nakayama (Tokai Chita Clinic); Masaya Shibata (Toyohashi Mates Clinic); Mitsuru Shimada (Toyooka Clinic); and Tetsuya Yamada (Toyota Kyoritsu Clinic).