Abstract
Hepcidin is a small defensin-like peptide, the production of which by hepatocytes is modulated in response to anemia, hypoxia, or inflammation. Kidneys are involved in not only the synthesis of hepcidin, but they also may be involved in its elimination. A cross-sectional study was performed to assess prohepcidin and hepcidin in serum, urine, and ultrafiltrate/peritoneal effluent in relation to type of renal replacement therapy and prohepcidin and hepcidin correlations with renal function, iron status, and markers of inflammation. Methods. Prohepcidin and hepcidin high-sensitivity CRP, TNF alpha, and IL-6 were measured using commercially available kits in 102 patients on hemodialyses, 17 on hemodiafiltration, 44 on peritoneal dialyses, and 22 healthy volunteers. Results. In hemodialyzed and peritoneally dialyzed patients with residual renal function, serum prohepcidin (264.21 ± 95.84 vs. 341.84 ± 90.45 ng/mL, p < 0.01; 142.76 ± 57.87 vs. 238.42 ± 84.32 ng/mL, p < 0.01, respectively) and hepcidin (178.89 ± 89.87 vs. 295.76 ± 129.65 ng/mL, p < 0.01; 108.43 ± 75.49 vs. 186.53 ± 119.62 ng/mL, p < 0.01, respectively) were significantly lower than in anuric patients. In peritoneal effluent, prohepcidin level was significantly higher than in ultrafiltrate of HD/HDF patients. In multiple regression analysis, residual renal function, ferritin, and hsCRP were predictors of hepcidin in hemodialyzed patients, while residual renal function and ferritin were predictors of hepcidin in peritoneally dialyzed patients. Conclusions. Residual renal function seems to play a pivotal role in hepcidin levels in dialyzed patients. In addition, the presence of low-grade inflammation, more pronounced in anuric patients, and functional iron deficiency may also contribute to the elevated hepcidin. The removal of prohepcidin with ultrafiltrate/peritoneal effluent may partially explain its lower concentration in peritoneal dialysis and hemodiafiltration.
INTRODUCTION
Maintaining the correct iron balance is crucial for health. Our understanding of the molecular control of iron metabolism has increased dramatically over the past five years due to the discovery of hepcidin. This is a circulating antimicrobial peptide that is synthesized in the liver. It has been recently proposed as a factor regulating the uptake of dietary iron, iron recycling by macrophages, and its mobilization from hepatic stores. However, it has been shown recently that the kidney is also involved in iron metabolism. Hepcidin, a recently discovered, small, cysteine-rich cationic peptide, was isolated from human urineCitation[1] and blood.Citation[2] Kulaksiz et al.Citation[3] suggested also that the kidneys, in addition to the liver, are involved not only in the synthesis but may participate in the hepcidin elimination as well.
The gene encoding hepcidin is also regulated by inflammation.Citation[4] Hepcidin constitutes an important link between iron metabolism, host defense, and inflammation. Hepcidin synthesis is markedly increased during infection and inflammationCitation[4] and reduces serum iron.Citation[4] During the inflammatory process, cytokines are primarily produced by macrophages and monocytes; these include interleukin (IL)-6, IL-1 beta, tumor necrosis factor-alpha (TNF alpha), interferon gamma, and transforming growth factor (TGF)-beta. These proteins stimulate acute phase protein production, with IL-6 being the major inducer of the majority of acute phase proteins.Citation[5] This family of cytokines also suppresses hepatic albumin synthesis.Citation[6] In a study on 46 patients with chronic renal failure who are not yet on dialysis, the serum hepcidin correlated with 51Cr-EDTA clearance (r = –0.44; p = 0.005), creatinine clearance, serum creatinine, beta-trace protein, and cystatin C.Citation[7] However, the authors did not provide biochemical characteristics of the studied population, not even a mean serum creatinine. In contrast to our study, no relationship was found between red cell indices or iron status and serum prohepcidin concentrations.Citation[8] Because significant correlations were observed between prohepcidin and kidney function, we focused on the role of kidney and residual renal function on prohepcidin metabolism.Citation[9] Our data—that creatinine and residual renal function in hemodialyzed patients and GFR in kidney transplant recipients are correlates of prohepcidin—partly support this hypothesis. We concluded that elevated prohepcidin in patients with chronic kidney disease, on hemodialysis, and kidney allograft recipients may be due to low-grade inflammation (as reflected by prohepcidin correlations with hsCRP, albumin, and ferritin), which is frequently encountered in this population, resulting from impaired renal function. A cross-sectional study was performed to assess prohepcidin in serum, urine, and peritoneal effluent/ultrafiltrate in HD in relation to type of dialyses (HD, PD) and prohepcidin correlations with renal function, iron status, and markers of inflammation in patients on hemodialyses, on hemodiafiltration, and on peritoneal dialyses.
PATIENTS AND METHODS
The study was performed on three groups of clinically stable dialyzed patients: 102 chronically hemodialyzed patients, 17 patients on hemodiafiltration, and 44 patients maintained on chronic ambulatory peritoneal dialysis (CAPD). Inclusion criteria were a stable clinical state, no thrombosis or inflammation (i.e., C-reactive protein within normal range, below 6 mg/L, using semiquantitative method), absence of acute cardiovascular complications (including uncontrolled hypertension, acute coronary syndrome, or acute heart failure), no oral contraception in women of childbearing age, and stable and no more than twice of the normal AspAT and AlAT activities. We did not include patients with renal graft failure and/or on immunosuppressive therapy. None of the patients investigated had received blood transfusions for at least 1.5 months prior the study.
All of the patients had required regular hemodialyses for 4–5 h a day three times a week (median time on HD was 30 months, range 3–241 months). Blood flow was usually 180–280 mL/min with a dialysate flow of 500 mL/min. Vascular access for HD was a native arterio-venous fistula on the forearm (n = 80) or on the arm (n = 10). Ultrafiltration varied according to patient's actual weight. All HD patients were dialyzed using low-flux polysulfone membranes (Fresenius, Bad Homburg, Germany) and low-flux modified cellulose membranes (Terumo, Althin, Gambro) with bicarbonate-buffered dialysate. Mean Kt/V was 1.19 ± 0.21. The causes of renal failure among HD patients varied between chronic glomerulonephritis (n = 34), chronic interstitial nephritis (n = 11), polycystic kidney disease (n = 11), diabetic nephropathy (n = 21), amyloidosis (n = 8), and others or unknown (n = 17). Seventy-nine patients were treated with recombinant human erythropoietin, 71 with antihypertensive drugs, and 80 patients received intravenous iron supplementation. Residual renal function was defined as passing more than 500 ml urine per day.
In the HDF patients, the end-stage renal failure was due to chronic glomerulonephritis (n = 9), chronic interstitial nephritis (n = 3), polycystic kidney disease (n = 2), and other or unknown causes (n = 3). Blood flow was usually 180–280 mL/min, and dialysate flow with online-HDF was 800 mL/min. All HDF patients were dialyzed using high-flux polysulfone membranes bicarbonate-buffered dialysate. Mean Kt/V was 1.25 ± 0.17. In the HDF group, the first modality treatment in the week was HDF, and then two sessions of HD. HDF was mainly offered to patients with hypotensive episodes during HD and/or poor tolerance of high ultrafiltration rate during HD.
In CAPD patients, renal failure was due to glomerulonephritis (n = 18), chronic interstitial nephritis (n = 10), polycystic kidney disease (n = 5), diabetic nephropathy (n = 7), and unknown cause (n = 4). The dialyzed patients were on CAPD for 1–5 years (median time on CAPD was 20 months, range 3–100 months). Thirty-two patients were treated with recombinant human erythropoietin and 31 with antihypertensive drugs. All of the patients were on oral iron supplementation. All of the CAPD patients were performing four 2 l exchanges a day. They used the Baxter or Fresenius system with conventional solutions (low pH, high concentration of glucose). Dwell times were generally 4–6 h during the day and 8 h overnight. The glucose concentration ranged from 1.36 to 3.86%. None of the patients was administered with icodextrin. The osmotic pressure of CAPD fluid was adjusted in accordance with the extent of ultrafiltration in each patient. Dialysis adequacy was assessed by measuring of Kt/V (mean Kt/v, 2.33 ± 0.41). In all CAPD patients, blood was drawn between 8:00 and 9:00 am when they appeared for routine examination at the outpatient unit after overnight fast. Blood was taken without stasis. Samples were aliquotted and stored at –40°C up to one month before assay. Samples of dialysate were also taken for prohepcidin assessment.
In all HD patients, blood was drawn between 8:00 and 9:00 am before the onset of midweek dialysis session (and heparin administration) and after hemodialysis from the arterial line of hemodialysis system immediately before discontinuation of the extracorporeal circulation (only for urea concentration necessary for Kt/V determination, a marker of adequacy of dialysis, mean Kt/V was 1.20 ± 0.21). As an anticoagulant, during hemodialysis, enoxaparin clexane (Aventis) was given as a single intravenous injection at the beginning of each dialysis session. The blood was centrifuged at 2500 g for 15 min at room temperature to serum. Samples were aliquotted and stored at –40°C before assay. Samples of ultrafiltrate were also taken for prohepcidin assessment. All patients were informed about the aim of the study and gave their consent. The study was approved by the local Medical University Ethic Committee. Control group consisted of 22 healthy volunteers (age range 29–72 years, 11F, 11M, with normal renal function [i.e., creatinine less than 1.2 mg/dL]) recruited mainly from the medical staff and their friends and families, all of whom were age- and sex-matched. High-sensitivity CRP was studied using kits from American Diagnostica (Greenwich, Connecticut, USA). Soluble receptor of transferrin-sTfR, interleukin-6 (high-sensitivity) and TNFα (high sensitivity) were studied using kits from R&D (Abington, UK). Prohepcidin was studied using a commercially available kit from DRG Instruments GmbH (Marburg, Germany), and hepcidin was estimated by radioimmunoassay from Bachem, UK. Inter- and intraassay variations were less than 10%.
Hemoglobin, hematocrit, total protein, cholesterol, HDL, LDL, triglycerides, albumin, CRP (for screening purposes, using a semiquantitative method in which values below 6 mg/L are considered normal and not calculated), calcium, phosphate, and creatinine were measured by standard laboratory methods in a central laboratory.
Statistical Analysis
Data given were analyzed using Statistica 6.0. computer software. If possible, data were logarithmically transformed to achieve normal distribution. Normality of variable distribution was tested using Shapiro-Wilk W-test. Measurements normally distributed are reported as mean ± SD, and non-normally distributed data are expressed as a median and minimal-maximal value. Analysis of variance (ANOVA) (with post hoc Tukey test for unequal groups) and Kruskall-Wallis ANOVA (the difference between the mean of two variables being calculated by Mann-Whitney U test) were used in statistical analysis to compare differences between groups, with p < 0.05 considered statistically significant, when appropriate. Linear regression analysis employed Pearson or Spearman coefficients as appropriate. Multiple regression analysis was used to determine independent factors affecting dependent variable. Factors showing linear correlation with prohepcidin (p < 0.1) were included in the analysis.
RESULTS
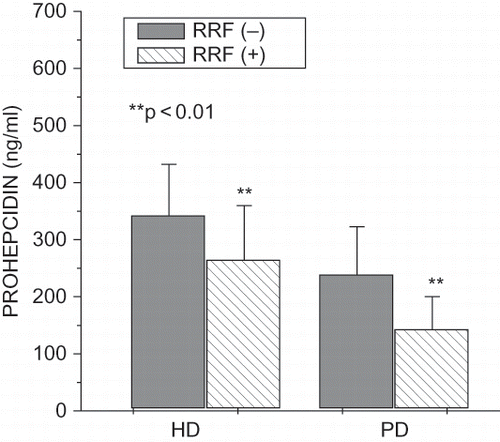
All of the clinical and biochemical data are shown in . Ferritin and prohepcidin were higher in hemodialyzed patients than in peritoneally dialyzed patients and HDF patients. In peritoneal effluent in PD patients, prohepcidin level was significantly higher than in ultrafiltrate of HD patients (see ). Among HD patients, 25 had hemoglobin levels less than 10 g/dL; in the HDF, group 6 patients had hemoglobin levels less than 10 g/dL; and only 4 PD patients had hemoglobin levels lower than 10 g/dL.
Table 1 Biochemical characteristics of hemodialyzed patients, patients on HDF, peritoneally dialyzed patients, and the control group
Table 2 Prohepcidin and hepcidin in PD, HD, HDF, and the control group
Effect of Hemodialysis on Prohepcidin and Hepcidin Levels
The effect of hemodialysis session on prohepcidin/hepcidin and values of prohepcidin in urine and ultrafiltrate (HD)/peritoneal effluent (PD) are shown in the .
HD and HDF significantly reduced hepcidin levels but not prohepcidin levels.
Impact of Residual Renal Function on the Studied Parameters
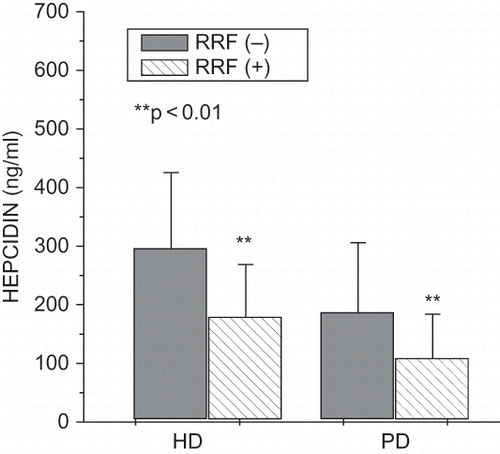
Hemodialyzed patients without residual renal function were dialyzed significantly longer than patients with residual renal function (59.62 ± 58.06 vs. 29.80 ± 28.62 months, p < 0.01). In hemodialyzed patients with residual renal function, we found significantly higher Kt/V (1.21 ± 0.30 vs. 1.02 ± 0.42, p < 0.05) and total protein (6.73 ± 0.49 vs. 6.53 ± 0.48 g/dL, p < 0.05), as well as significantly lower serum creatinine (7.17 ± 2.18 vs. 9.40 ± 2.93 mg/dL, p < 0.01), fibrinogen (334.6 ± 90.7 vs. 381.5 ± 81.6 mg/mL, p < 0.05), serum prohepcidin (264.21 ± 95.84 vs. 341.84 ± 90.45 ng/mL, p < 0.01; see ), hepcidin (178.89 ± 89.87 vs. 295.76 ± 129.65 ng/mL, p < 0.01; see ), IL-6 (1.85 ± 2.05 vs. 4.17 ± 5.22 pg/mL, p < 0.001), hsCRP (3.76 ± 2.65 vs. 7.19 ± 4.93 mg/L, p < 0.001), and ferritin (254.41 ± 181.18 vs. 413.83 ± 301.43 ng/mL, p < 0.01). Peritoneally dialyzed patients without residual renal function were dialyzed significantly longer than patients with residual renal function (50.40 ± 33.10 vs. 20.10 ± 15.81 months, p < 0.01). In peritoneally dialyzed patients with residual renal function, we found a significantly higher Kt/V (2.24 ± 0.34 vs. 1.99 ± 0.49, p < 0.01), cholesterol (221.73 ± 47.98 vs. 162.20 ± 33.16 mg/dL, p < 0.01), and LDL (133.83 ± 40.35 vs. 89.00 ± 38.81 mg/dL, p < 0.05), as well as significantly lower serum creatinine (7.34 ± 2.32 vs. 8.98 ± 2.51 mg/dL, p < 0.01) and serum prohepcidin (142.76 ± 57.87 vs. 238.42 ± 84.32 ng/mL, p < 0.01; see ), hepcidin (108.43 ± 75.49 vs. 186.53 ± 119.62 ng/mL, p < 0.01; see ), hsCRP (3.02 ± 2.91 vs. 9.76 ± 8.05 mg/L, p < 0.001), and ferritin (217.89 ± 165.87 vs. 419.21 ± 291.01 ng/mL, p < 0.01).
Correlations between Hepcidin/Prohepcidin and Parameters Studied
Hepcidin correlated negatively with albumin (r = –0.30, p < 0.05), hemoglobin (r = –0.22, p < 0.05), triglycerides (r = –0.24, p < 0.05), and LDL (r = –0.24, p < 0.05), and positively with hsCRP (r = 0.22, p < 0.05) and erythropoietin dose (r = 0.23, p < 0.05) in hemodialyzed patients. In multiple regression analysis, residual renal function (beta value was –0.47. p = 0.02), ferritin (beta value was 0.35, p = 0.03), and hsCRP (beta value was beta 0.36, p = 0.04) were predictors of hepcidin in hemodialyzed patients. It explains 44% of the variations of hepcidin concentration. Multiple adjusted r2 = 0.44, F = 2.63, p < 0.02, SE = 116.15.
Urinary prohepcidin correlated significantly with albumin (r = –0.28, p < 0.05), hsCRP (r = 0.43, p < 0.01), serum prohepcidin (r = 0.41, p < 0.01), and aspartate aminotransferase (r = 0.35, p < 0.05). Prohepcidin in the ultrafiltrate correlated significantly with total protein (r = –0.39, p < 0.05) and albumin (r = 0.30, p < 0.05).
Hepcidin correlated positively with albumin (r = 0.42, p < 0.01), ferritin (r = 0.31, p < 0.05), iron (r = 0.29, p < 0.05), hsCRP (0.37, p < 0.05) in peritoneally dialyzed patients. In multiple regression analysis, residual renal function (beta value was –0.41. p = 0.02) and ferritin (beta value was 0.39. p = 0.049) were predictors of hepcidin in peritoneally dialyzed patients. It explains 26% of the variations of hepcidin concentration. Multiple adjusted r2 = 0.26, F = 3.71, p < 0.02, SE = 40.50.
Urinary and peritoneal effluent prohepcidin did not correlate significantly with the studied parameters in peritoneally dialyzed patients.
In HDF, prohepcidin and hepcidin did not correlate significantly with the studied parameters. In the healthy volunteers, prohepcidin was related to ferritin (r = 0.50, p < 0.01). Hepcidin and prohepcidin did not correlate with each other in HD, HDF, PD, and healthy volunteers.
DISCUSSION
Deteriorating renal function may enhance overall inflammatory responses because of the decreased renal clearance of factors that are directly or indirectly involved in inflammation. As an example, the serum half-lives of pro-inflammatory cytokines, tumor necrosis factor alpha and interleukin-1, are greater in animals without renal function.Citation[10] A declining renal function may also affect the levels of additional inflammatory molecules, such as serum C-reactive protein (CRP) or interleukin-6, which are inversely correlated with creatinine clearance.Citation[11,Citation12] Nemeth et al.Citation[13] observed that urinary excretion of hepcidin increased significantly in patients with hypoferremia and anemia due to infections or inflammatory diseases. In human volunteers, IL-6 infusion caused hypoferremia (fall by 34%) within two hours as well as a 7.5-fold increase in urinary hepcidin.Citation[14] In addition, in dialysis patients with residual renal function, higher serum CRP concentrations and IL-6 are observed among those with relatively less native kidney function.Citation[15,Citation16] In our previous study, we observed that in patients with CRP ≥6 mg/L, hepcidin was significantly higher than in patients with CRP less than 6 mg/L. In multiple regression analysis, prohepcidin was independently related to albumin and triglycerides in hemodialyzed patients.Citation[17] In a study on 46 patients with chronic renal failure not yet on dialysis, the serum hepcidin correlated with 51Cr-EDTA clearance (r = –0.44; p = 0.005), creatinine clearance, serum creatinine, beta-trace protein, and cystatin C.Citation[8] However, the authors did not provide biochemical characteristics of the studied population, not even a mean serum creatinine. In contrast to our study, no relationship was found between red cell indices or iron status and serum prohepcidin concentrations.Citation[8] Kulaksiz et al.Citation[3] also suggested that kidneys, in addition to the liver, are not only involved in the synthesis, but may participate in the hepcidin elimination as well. They reported that hepcidin was produced as an intrinsic peptide in the epithelial cells of the tubule and collecting duct in mammalian kidney and might be released luminally into the urine.Citation[3] Our findings that creatinine and residual renal function in hemodialyzed patients and GFR in kidney transplant recipients are correlates of hepcidin partly support this hypothesis.
Because significant correlations were observed between prohepcidin and kidney function, we also focused on the role of kidney and residual renal function on prohepcidin metabolism.Citation[9] Our findings that creatinine and residual renal function in hemodialyzed patients and GFR in kidney transplant recipients are correlates of prohepcidin partly support this hypothesis. Tomosugi et al.Citation[18] recently employed the ProteinChip System to detect serum hepcidin in renal failure. A new technology, the surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS), is based on classical solid phase extraction chromatography combined with direct laser desorption/ionization mass spectrometric detection. They developed a semiquantitative assay system to detect serum hepcidin-25 to tackle and overcome the technical problems of hepcidin determination in dialyzed patients. They found that in two hemodialysis patients they studied, the intensities of two peaks corresponding to hepcidin-20 and hepcidin-25 were higher than in healthy volunteers. These forms of hepcidin were sufficiently cationic to be filtered through the anionic glomerular membrane. They also reported that hepcidin-25 did not fall after the dialysis session in some patients. In one recent study, Ashby et al.Citation[19] showed no reduction in hepcidin following standard hemodialysis session in samples taken from six patients using novel competitive immunoassay (68.7 vs. 69.7 ng/mL pre-and post-dialysis, respectively); however, HD patients characteristics was not presented, and data on residual renal function, dialyzers type, etc. are missing. They also found an inverse correlation between hepcidin and eGFR in 44 patients with chronic kidney disease (r = –0.53, p = 0.0002), suggesting a role of kidney in hepcidin elimination. In addition, they identified no correlation between hepcidin and IL-6 or CRP in both HD and CKD population. In their study, residual renal function has no effect on hepcidin levels. They did not provide any data on the types of dialyzers used. In the study of Kato et al.,Citation[20] characteristics of the dialyzer membrane did not affect the prohepcidin and hepcidin 25 intensity. They used the following membranes: high-flux polysulfone hollow fiber (BS-U, Toray, Asahi Medical, Japan or FPX, Fresenius, Germany), cellulose triacetate (FB-U, Nipro Medical, Japan), ethylene-vinyl alcohol (Kf-m, Kuraray Medical, Japan), or polyester-polymer alloy membrane (FDY-GW, Nikkiso Co., Japan). We also did not find any statistically significant changes in prohepcidin and hepcidin in regard to the type of dialyzers used (F5, F6, F60, GFE 09, GFS plus, Althin Ultra Nova 140, AV160S, BL621, FB130T).
Hepcidin and prohepcidin did not correlate with each other in HD, HDF, PD, and healthy volunteers. Due to many inconsistencies in hepcidin measurements, we assessed simultaneously prohepcidin and hepcidin and found the similar tendency in changes in both parameters; however, hepcidin and prohepcidin were unrelated. (Note that this is the first report to use Bachem RIA assay to detect hepcidin.) Because prohepcidin levels do not appear to be physiologically relevant,Citation[21] hepcidin should be measured once sensitive and specific commercially available enzyme- or radioimmunoassays are developed and validated.
At present, there is no consensus on the best assay for hepcidin measurement, and assays for hepcidin detection and quantification in serum or urine have not been generally available. The detection and quantification of hepcidin in plasma and serum have been hampered by technical difficulties (e.g., the small size of hepcidin, which is a 25 amino acid peptide with molecular weight about 2.7 kDa, while prohepcidin consists of 60 amino acids with molecular weight about 10 kDa; limited availability of the antigen; the isolation of hepcidin from urine involving complex, time-consuming procedures). There are still no reliable data available on normal serum levels of mature hepcidin (20, 22, and 25 amino acids). Other recent serum hepcidin assays based on the surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS), liquid chromatography tandem mass spectrometry (LC-MS/MS, and isotope dilution micro-HPLC-tandem mass spectrometry.Citation[22–24] In addition to an ELISA assay for mature hepcidin measurement developed by Ganz et al.,Citation[25] two more competitive immunoassays using anti-hepcidin 25 antibodies have been reportedCitation[19,Citation26] as well as novel RIA.Citation[27] However, analytical detection limit of their ELISA and normal ranges are different between these assays, as recently reviewed.Citation[28] Due to many inconsistencies in hepcidin measurements, we assessed simultaneously prohepcidin and hepcidin (using Bachem RIA assay to detect mature hepcidin); however, hepcidin and prohepcidin were unrelated. RIA is commercially available; therefore, it would be of interest to compare competitive immunoassays developed by others, particularly in regard to their possible commercial availability. Because prohepcidin levels do not appear to be physiologically relevant,Citation[21] hepcidin should be measured once sensitive and specific commercially available enzyme- or radioimmunoassay is confirmed and validated.
In conclusion, in our study, residual renal function seems to play a pivotal role in hepcidin levels in dialyzed patients. In addition, the presence of low-grade inflammation, more pronounced in anuric patients, and functional iron deficiency may also contribute to the elevated hepcidin. Removal of prohepcidin with ultrafiltrate /peritoneal effluent may partially explain its lower concentration in peritoneal dialysis and hemodiafiltration.
ACKNOWLEDGMENTS
The studies were supported by a research grant from the Medical University of Bialystok, Poland.
References
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810.
- Krause A, Neitz S, Magert HJ Schyltz A, Forssmann WG, Schulz-Knappe P, Adermann L. LEAP-1, a novel highly disulfide-bonded human peptide exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150.
- Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, Stremmel W. Prohepcidin: Expression and cell specific localization in the liver and its regulation in hereditary hemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735–743.
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044.
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon b2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255.
- Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanisms of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79:1635–1641.
- Taes YE, Wuyts B, Boelaert JR, De Vriese AS, Delanghe JR. Prohepcidin accumulates in renal insufficiency. Clin Chem Lab Med. 2004;42:387–389.
- Allen DA, Breen C, Yaqoob MM, Macdougall IC. Inhibition of CFU-E colon formation in uremic patients with inflammatory disease. Role of IFN-gamma and TNF-alpha. J Investig Med. 1999;47:204–211.
- Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M. Hepcidin, iron status and renal function in chronic renal failure, kidney transplantation and hemodialysis. Am J Hematol. 2006;81:832–837.
- Poole S, Bird TA, Selkirk S, Gaines-Das RE, Choudry Y, Stephenson SL, Kenny AJ, Saklatvaa J. Fate of injected interleukin 1 in rats: Sequestration and degradation in the kidney. Cytokine. 1990;2:416–422.
- Panichi V, Migliori M, De Pietro S, Taccola D, Bianchi AM, Norpoth M, Metelli MR, Giovannini L, Tetta C, Palla R. C reactive protein in patients with chronic renal diseases. Ren Fail. 2001;23:551–562.
- Stenvinkel P, Heimburger O, Wang T, Lindholm B, Bergstrom J, Elinder CG. High serum hyaluronan indicates poor survival in renal replacement therapy. Am J Kidney Dis. 1999;34:1083–1088.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463.
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276.
- Chung SH, Heimburger O, Stenvinkel P, Bergstrom J, Lindholm B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant. 2001;16:2240–2245.
- Pecoits-Filho R, Heimburger O, Barany P, Suliman M. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218.
- Malyszko J, Malyszko JS, Hryszko T, Pawlak K, Mysliwiec M. Is hepcidin a link between anemia, inflammation and liver function in hemodialyzed patients?. Am J Nephrol. 2005;25:586–590.
- Tomosugi N, Kawabata H, Wakatabe R, Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387.
- Ashby DR, Gale DP, Busbridge M, Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009 Feb 11 [E-pub ahead of print].
- Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008;28:115–121.
- Roe MA, Spinks C, Heath AL, Harvey LJ, Foxall R, Wimperis J, Wolf C, Fairweather-Tait SJ. Serum prohepcidin concentration: No association with iron absorption in healthy men; and no relationship with iron status in men carrying HFE mutations, hereditary hemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97:544–549.
- Murphy AT, Witcher DR, Luan P, Wroblewski VJ. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110:1048–1054.
- Kobold U, Dulffer T, Dangl M, Escherich A, Kubbies M, Röddiger R, Wright JA. Quantification of hepcidin-25 in human serum by isotope dilution micro-HPLC-tandem mass spectrometry. Clin Chem. 2008;54:1584–1586.
- Li H, Rose MJ, Tran L, Zhang J, Miranda LP, James CA, Sasu BJ. Development of a method for the sensitive and quantitative determination of hepcidin in human serum using LC-MS/MS. J Pharmacol Toxicol Methods. 2009;59:171–180.
- Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297.
- Koliaraki V, Marinou M, Vassilakopoulos TP, Vavourakis E, Tsochatzis E, Pangalis GA, Papatheodoridis G, Stamoulakatou A, Swinkels DW, Papanikolaou G, Mamalaki A. A novel immunological assay for hepcidin quantification in human serum. PLoS ONE. 2009;4:e4581.
- Grebenchtchikov N, Geurts-Moespot AJ, Kroot JJ, den Heijer M, Tjalsma H, Swinkels DW, Sweep FG. High-sensitive radioimmunoassay for human serum hepcidin. Br J Haematol 2009 Jun 3. PubMed PMID: 19500086 [E-pub ahead of print].
- Malyszko J. Hepcidin assay-ironing out the details. Clin J Am Soc Nephrol. 2009;4:1015–1016.