Abstract
Background/Aims. Different serum and urinary biomarkers have been recently proposed to serve as markers of acute kidney injury. We tested the hypothesis whether NGAL and other biomarkers could represent an early biomarker of contrast nephropathy (CIN) in diabetic patients with normal serum creatinine undergoing cardiac catheterization in comparison with non-diabetic patients. Methods. Serum, urinary NGAL, cystatin C, urinary kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), and liver-type fatty acid binding protein (L-FABP) were evaluated before and 2, 4, 8, 24, and 48 hours after cardiac catheterization using commercially available kits. Results. In both groups we found a significant rise in serum NGAL after 2, 4, and 8 hours, and in urinary NGAL and IL-18 after 4, 8, and 24 hours after cardiac catheterization. Serum cystatin C increased significantly 8 hours, reaching peak 24 hours after cardiac catheterization in both groups, and then decreased after 48 hours. L-FABP and KIM-1 increase significantly after 24 and 48 hours after cardiac catheterization. Conclusions. CIN was similarly prevalent in both diabetic and non-diabetic patients undergoing cardiac catheterization. NGAL seems to be a potential early marker for nephrotoxicity and predictor of contrast nephropathy. It is particularly important in the upcoming setting of short-time hospitalizations for cardiac catheterization.
INTRODUCTION
It has been estimated that by the year 2030, 366 million people worldwide will have diabetes.Citation[1] Greater than 5% of newly diagnosed patients with type 2 diabetes will already have diabetic kidney disease, and a further 30–40% will develop diabetic nephropathy, mostly within 10 years of diagnosis.Citation[2] It is increasingly appreciated that diabetes and chronic renal dysfunction alone are independent factors for the development of coronary artery disease. In addition, patients with CKD and/or diabetes have an enhanced mortality after an acute coronary syndrome and after percutaneous coronary interventions (PCI) with or without stenting. Because interventional cardiologists are being asked more frequently to perform PCI on increasing numbers of patients with significant co-morbidities such as CKD and/or diabetes, contrast nephropathy is a potentially serious complication of PCI.Citation[3] Most commonly, it is defined as an acute impairment of renal function manifested by an absolute increase in serum creatinine of at least 0.5 mg/dL or by a relative increase by at least 25% from the baseline levels. Peak creatinine typically occurs 3–5 days after contrast administration and returned to baseline (or a new baseline) in 1–3 weeks,Citation[3] when patients are discharged from the hospital. Because creatinine is an unreliable indicator during acute changes in kidney function,Citation[3] the search for new biomarkers of acute kidney injury continues. The value of neutrophil gelatinase-associated lipocalin (NGAL) was highlighted as a novel biomarker of detection of acute renal failure, compared even to cardiac troponin.Citation[4] Because of its small molecular size (25 kDa) and resistance to degradation, NGAL is readily excreted and detected in urine. NGAL is highly accumulated in the human kidney cortical tubules, blood, and urine after nephrotoxic and ischemic injury.Citation[5] Besides NGAL, various molecular biomarkers are currently under investigation to determine their value as indicators of renal injury. Among the other top candidates at present are interleukin 18 (IL-18), kidney injury molecule-1 (KIM-1), and urinary liver-type fatty acid-binding protein (L-FABP).Citation[6,Citation7]
While it has been clearly demonstrated that the presence of CKD and diabetes is a risk factor for radiocontrast-associated nephrotoxicity that can result in decreased short- and long-term survival,Citation[8,Citation9] the relationship between kidney function assessed by serum creatinine, cystatin C, and NGAL and other urinary biomarkers; presence of diabetes; and cardiac procedures is yet to be defined. In this study, we tested the hypothesis whether NGAL could represent an early biomarker of contrast-induced nephropathy (CIN) in 70 diabetic patients with stable angina and normal serum creatinine undergoing cardiac catheterization with or without PCI in comparison to 70 sex- and age-matched non-diabetic patients. Second, we assessed serum and urinary NGAL in relation to cystatin C, eGFR serum, and urinary creatinine in these patients. Third, we examined changes in other urinary biomarkers of renal injury as KIM-1, L-FABP, and IL-18 in this population.
PATIENTS AND METHODS
Methods
The study was performed on 140 Caucasians patients (i.e., 70 type 2 diabetic patients in comparison to 70 sex- and age-matched non-diabetic patients) with normal serum creatinine undergoing cardiac catheterization with or without PCI to stable angina (II/III class according to CCS).
The decision of performing either coronary angiography (n = 92) or angioplasty with stenting (n = 48) (i.e., coronary angiography and subsequent PCI) was based on the decision of the invasive cardiologist during the procedure. We excluded patients with preexisting kidney disease (eGFR according to MDRD below 60 mL/min). We decided to study only low-risk patients (i.e., with stable angina and without severe kidney disease) due to the fact that high-risk patients with concomitant comorbidities (e.g., chronic kidney disease, diabetic nephropathy, advanced chronic heart failure) are generally given much attention and were the subject of the previous studies.Citation[7,Citation8] None of the patients investigated had received nephrotoxic drugs for at least one week before and during the study period. All the patients were informed about the aim of the study and gave their consent, and the protocol was approved by the local Ethics Committee. All clinical and biochemical data are given in . Among all type 2 diabetic patients studied, 31 were treated with insulin and the rest with oral hypoglycemic drugs. In all patients, 24 hours before cardiac catheterization, all nephrotoxic drugs (i.e., NSAIDs, diuretics, biguanidine derivatives in diabetic patients) were withdrawn and ACE inhibitors were either withdrawn (when blood pressure permitted) or halved 24 hours before the procedure. All patients admitted to the Department of Invasive Cardiology were given 2 liters of hydration within 24 hours periprocedurally. Contrast nephropathy (CIN) was defined as an increase in serum creatinine by >25% of the baseline level within 48 hours after cardiac catheterization.
Table 1 Basal clinical and biochemical characteristic of the studied patients
Iso- or low-osmolal contrast agent (iodixanol n = 24 or iopromide n = 116) was used in all of the studied patients. Serum and urinary NGAL, urinary KIM-1, L-FABP, and IL-18 were evaluated before and after 2, 4, 8, 24, and 48 hours after cardiac catheterization. Serum cystatin C and serum creatinine were assessed before cardiac catheterization 24 and 48 hours after the procedure. Hemoglobin, hematocrit, HbA1C, cholesterol, HDL, triglycerides, fasting glucose, blood pressure, creatinine, ejection fraction, and left ventricular internal end-diastolic dimension (LVIDd) were studied at admission. We assessed kidney function according to the simplified MDRD equation:
Citation[10]
as well as the Cockcroft-GaultCitation[11] and JeliffeCitation[12] formulas. NGAL was evaluated using commercially available ELISA from ANTIBODYSHOP (Gentofte, Denmark). Serum cystatin C was measured using commercially available kits from Dade Behring, Marburg, Germany. Urinary KIM-1 was assessed using commercially available kits from USNCB Life Co., Ltd, China; L-FABP using kits from CMIC Co., Ltd., Japan; and IL-18 using kits from R&D, Abington, UK.
All tests were performed according to manufactures' instructions by the same person. Data given were analyzed using Statistica 6.0 PL. ANOVA or Kruskall-Wallis ANOVA for repeated measurements were used in statistical analysis, with p < 0.05 considered statistically significant, when appropriate. Multiple regression analysis was used to determine independent factors affecting dependent variable. Factors showing linear correlation with NGAL (p < 0.1) were included in the analysis.
RESULTS
Baseline Characteristics
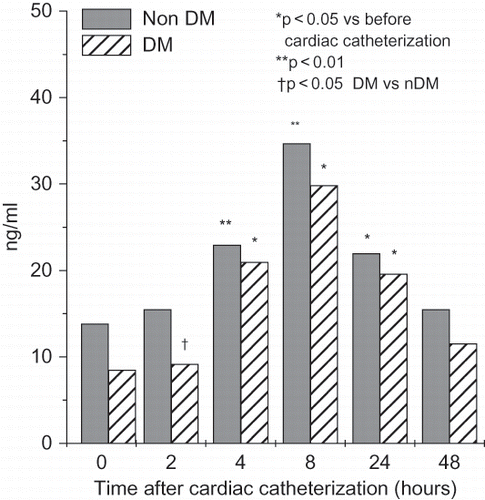
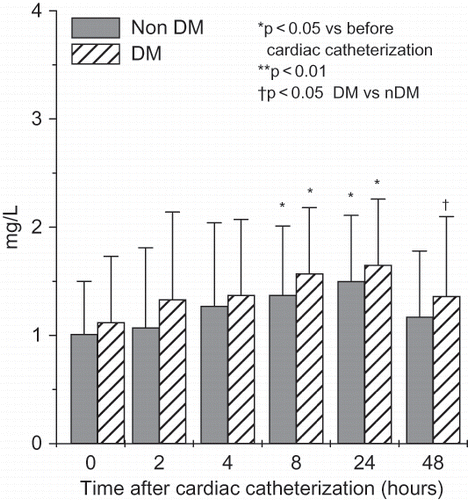
In both groups, we found a significant rise in serum NGAL after 2, 4, and 8 hours (see ), and in urinary NGAL after 4, 8, and 24 hours after cardiac catheterization (see ). Serum NGAL was significantly higher in diabetic patients 2, 4, 8, and 24 hours after PCI, whereas urinary NGAL was significantly higher 4, 8, and 24 hours after cardiac catheterization. We found a significant rise in serum NGAL after 2, 4, and 8 hours, and in urinary NGAL after 4, 8, and 24 hours after cardiac catheterization in non-diabetic patients. Serum cystatin C increased significantly after 8 hours, reaching its peak 24 hours after cardiac catheterization in both groups, and decreased after 48 hours (see ).
Figure 1. Time-course changes in serum NGAL (means ± SD) in non-diabetic and diabetic patients undergoing cardiac catheterization.
Univariate Analysis
Time-course changes in KIM-1, IL-18, and L-FABP are given in the Before cardiac catheterization serum, NGAL was related, in univariate analysis, to serum creatinine (r = 0.30, p < 0.05), urea (r = 0.39, p < 0.01), urinary NGAL (r = 0.28, p < 0.05), age (r = 0.31, p < 0.05), eGFR by MDRD (r = –0.41, p < 0.001), Cockcroft-Gault (r = –0.39, p < 0.01), Cockcroft-Gault weight-adjusted (r = –0.42, p < 0.001), Jeliffe (r = –0.37, p < 0.01), and cystatin C (r = 0.41, p < 0.001) in non-diabetic patients, but not with KIM-1, L‐FABP, and IL-18.
Table 2 Time-course changes urinary KIM-1, L-FABP, and IL-18 in diabetic and non-diabetic patients undergoing cardiac catheterization
In diabetic patients, serum NGAL was related in univariate analysis to serum creatinine (r = 0.32, p < 0.05), urea (r = 0.35, p < 0.01), urinary NGAL (r = 0.37, p < 0.01), age (r = 0.32, p < 0.05), hemoglobin (r = 0.40, p < 0.01), hematocrit (r = 0.40, p < 0.01), eGFR by MDRD (r = –0.43, p < 0.001), Cockcroft-Gault weight-adjusted (r = –0.39, p < 0.01), Jeliffe (r = –0.36, p < 0.01), and cystatin C (r = 0.44, p < 0.001) before cardiac catheterization, but not with KIM-1, L-FABP, and IL-18.
Multiple Regression Analysis
To the model of multiple regression analysis, parameters that correlated or tended to correlate with NGAL (p < 0.1) were included. We analyzed separately dependent variables describing kidney function. eGFR (MDRD) better explained NGAL variability than other variables (creatinine, cystatin C, creatinine clearance according to Cockcroft-Gault formula) in the model of multiple regression analysis. It was the only predictor of serum NGAL before cardiac catheterization (beta value, 0.49; p = 0.001). Multiple adjusted r2 for variables in the equation = 0.56, F = 8.38, p < 0.00001, SE of estimate = 43.87.
Impact of Type of Contrast Agent
Diabetic patients administered low-osmolal contrast (n = 11) had significantly higher urinary NGAL (median 18 [02.–189] vs. 40 [0.9–259] ng/mL, p < 0.05) four hours after PCI when compared to diabetic patients administered iso-osmolal contrast agent (n = 59). There were no statistically significant differences between other biomarkers in regard to type of the contrast agent used. In the non-diabetic population, no statistically differences in serum and urinary biomarkers were observed between patients administered iodixanol and iopromide.
Impact of Contrast-Induced Nephropathy (CIN) on NGAL, Cystatin C, and Other Urinary Biomarkers
When contrast nephropathy was defined as an increase in serum creatinine by >25% of the baseline level within 48 hours after cardiac catheterization, the prevalence of CIN was 10% in non-diabetics and 14% in diabetics (all patients receiving low-osmolal contrast). Patients with CIN received significantly more contrast agent (154.8 ± 77.6 ml vs. 205.0 ± 89.7 ml, p < 0.01), but duration of cardiac catheterization was not significantly different. All of them underwent coronary angioplasty with stenting.
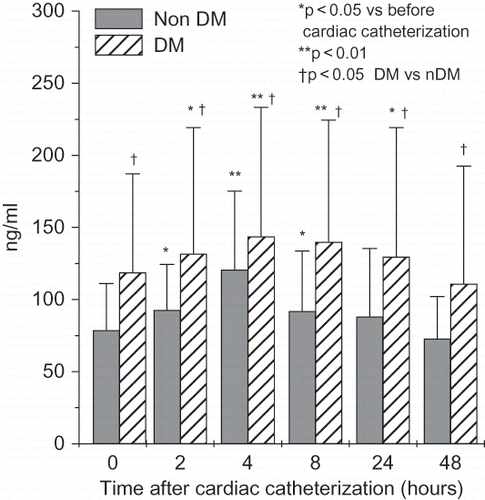
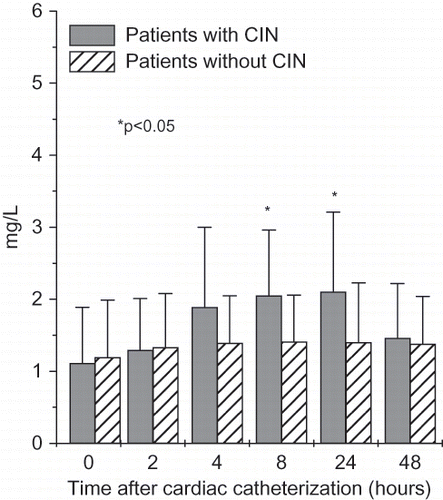
NGAL levels were significantly higher in patients with CIN starting two hours after cardiac catheterization (serum NGAL) (see ) or four hours (urinary NGAL). Even after 48 hours, serum and urinary NGAL were significantly higher in patients with CIN when compared to patients without CIN. Cystatin C was higher only 8 and 24 hours after cardiac catheterization in patients with CIN (see ). IL-18 followed cystatin C pattern and was significantly higher in patients with CIN 8 and 24 hours after cardiac catheterization, whereas L-FABP was significantly higher only 24 hours after the procedure. KIM-1 was tended to be statistically higher after 24 and 48 hours, though the difference did not reach statistical significance.
Figure 4. Time-course changes in serum NGAL (means ± SD) in all patients with and without contrast-induced nephropathy undergoing cardiac catheterization.
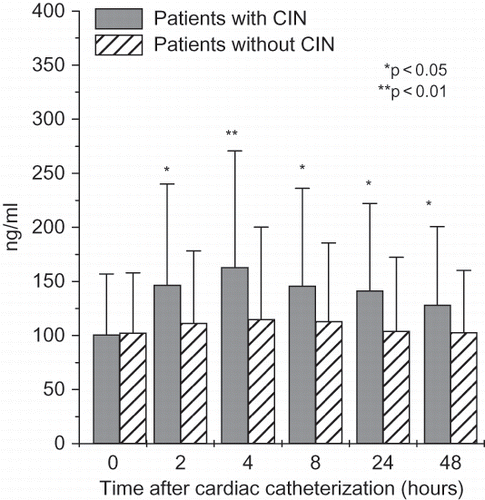
Figure 5. Time-course changes in serum cystatin C (means ± SD) in all patients with and without contrast-induced nephropathy undergoing cardiac catheterization.
When we defined contrast nephropathy as an absolute increase in serum creatinine from baseline of ≥0.5 mg/dL at 48 hours, we found only two cases of contrast nephropathy in diabetic patients.
Impact of Contrast-Induced Nephropathy (CIN) Defined as a Rise in Cystatin C by 25% from the Baseline
When we defined contrast nephropathy as a 25% increase in cystatin C from baseline values (n = 51), we then calculated sensitivity and specificity of a respective 25% serum and urinary NGAL increase to detect this cystatin C increase. We found 90% sensitivity and 74% specificity of serum and 76% sensitivity and 80% specificity of urinary NGAL increase, respectively. In patients with a 25% increase in serum cystatin C, we found a significantly higher serum and urinary NGAL (all p < 0.05) and higher cystatin C at baseline (p < 0.05) when compared to patients without 25% increase in serum cystatin C.
DISCUSSION
In our study, we found a similar prevalence of CIN in diabetic patients over non-diabetic patients with normal serum creatinine (10% vs. 14%). At least 5 percent of patients who undergo cardiac catheterization experience a transient rise in the plasma creatinine concentration of more than 1.0 mg/dL (88 μmol/L) due to contrast-induced renal dysfunction.Citation[13] The risk is negligible with normal renal function, even if the patient is diabetic,Citation[14] whereas it exceeds 50 percent or more, particularly in patients with diabetic nephropathy. Therefore, we chose a low-risk population and sought for a new biomarker of kidney dysfunction following contrast administration, bearing in mind that CIN was the third leading cause of hospital-acquired acute kidney injury, and optimal therapy to prevent this complication remained uncertain.Citation[15] On the other hand, very few biomarkers exist for monitoring chronic kidney disease. Because creatinine is not a sensitive marker of kidney function, in our study, we tried to assess whether NGAL could represent a novel and sensitive biomarker of kidney function. Simultaneously, we assessed serum creatinine, eGFR and NGAL and other urinary biomarkers in both groups of patients and found that serum NGAL was elevated diabetic patients relative to non-diabetic counterparts. We found that serum NGAL increased significantly in both groups after 2, 4, and 8 hours, and in urinary NGAL after 4, 8, and 24 hours after cardiac catheterization.
Mishra et al.Citation[16] found a rise in serum and urinary NGAL in samples taken after two hours or in the first available sample after cardiopulmonary bypass (CPB) in children who developed acute kidney injury (AKI). Children without AKI have a small but significant rise in serum NGAL at the same time. Therefore, we also chose two hours to sample blood and urine. The mechanisms of AKI in this setting are the ischemia-reperfusion, whereas the underlying pathology of contrast-induced renal failure is acute tubular necrosis. However, the mechanism is not well understood,Citation[17] with renal vasoconstriction resulting in medullary hypoxemia and direct cytotoxic effects of the contrast agents considered. The nephrotoxic properties of these agents appear to vary, with low- and iso-osmolal agents being associated with a relatively lower incidence of renal injury among high-risk patients. The 2007 focused update of the ACC/AHA/SCAI 2005 PCI guideline recommended the use of iso-osmolal in preference to low osmolal agents in patients with chronic kidney disease.Citation[18] In our study, two kinds of contrast agents were used, iso-osmolal and low-osmolal. All patients who developed CIN received low-osmolal agent. Some, but not all, studies have demonstrated a dose-dependent risk of renal dysfunction, with lower doses of contrast being safer (but not free of risk).Citation[19] Low dose has been variably defined as less than 70 mL, less than 125 mL, or less than 5 mL/kg (to a maximum of 300 mL) divided by the plasma creatinine concentration. However, diabetic patients with a plasma creatinine concentration above 5 mg/dL (440 μmol/L) may be at risk from as little as 20 to 30 mL of contrast.Citation[19] In our study, amount of contrast agent administered was significantly higher than in patients without CIN, whereas duration of the procedure did not differ significantly. There are few data on NGAL as a predictor of CIN. In the study of Hirsch et al.Citation[20] performed in 91 children with congenital heart disease undergoing elective cardiac catheterization and angiography with contrast administration, significant elevation of NGAL concentrations in urine and plasma were noted within 2 h after contrast administration. They reported that patient demographics and contrast volume were not predictive of CIN. Diabetic children were enrolled in their study, but their findings are in line with our results. In another study published by Ling et al.Citation[21] on patients undergoing coronary angiography using low-osmolar contrast medium, urine samples for NGAL and IL-18 assessment were collected before and 24 h after coronary angiography. At 24 h after the procedure, the urinary IL-18 and NGAL levels were significantly increased in the CIN group (13 patients out of 150, prevalence of CIN 8.7%), but not in the control group (p < 0.05, 27 patients). The predictable time of AKI onset determined by IL-18 was 24 h earlier than determined by serum creatinine (p < 0.01). We also found that IL-18 increased significantly 2 hours after cardiac catheterization in diabetic patients and 4 hours after PCI in non-diabetic patients, peaking at 24 hours in both groups. There are no data in the literature on such time course changes of IL-18, KIM-1, and L-FABP in contrast nephropathy as in our study. However, in the study of Parikh et al.,Citation[22] urine IL-18 increased 4–6 h after cardio-pulmonary bypass, peaked at over 25-fold at 12 h, and remained markedly elevated up to 48 h after cardio-pulmonary bypass. They suggested that IL-18 is an early, predictive biomarker of acute kidney injury after cardio-pulmonary bypass, and that NGAL and IL-18 are increased in tandem after cardio-pulmonary bypass. We studied a different model of acute kidney injury-contrast nephropathy and different population. On the other hand, Bulent Gul et al.Citation[23] studied urinary IL-18 values before and 24 and 72 h after the PCI. They found no statistically significant differences in urine IL-18 between cases of CIN (n = 15) and controls (n = 36) or between the patient samples obtained before PCI and after the invasive procedure in both study groups. They concluded that their findings argued against the hypothesis that urine IL-18 might be clinically useful as a biomarker of CIN after radiological procedures requiring intravascular administration of iodinated contrast media.
Kato et al.Citation[24] studied changes in creatinine, cystatin C, α,β microglobulins, NAG, and L-FABP in 87 patients undergoing elective catheterization with or without PCI. They found that L-FABP increased on days 1 and 2 after the procedure in 31 Japanese patients with stage 3 CKD, where prevalence of CIN was 42%. They also found a rise in L-FABP one day after the procedure in 41 patients with mild renal disease, defined as eGFR 60–89 mL/min (modified MDRD equation for Japanese). In our population, we observed a significant rise in L-FABP 24 and 48 hours after PCI in both diabetic and non-diabetic groups without any significant changes 2–8 hours after the procedure. Nakamura et al.Citation[25] studied 66 patients with serum creatinine between 1.2 and 2.5 mg/dL. They defined contrast medium nephropathy as an increase in serum Cr level of greater than 0.5 mg/dL or a relative increase of more than 25% at 2 to 5 days after the procedure and found that prevalence of CIN was almost 20%. They observed a rise in urinary L-FABP levels the next day and two days after angiography in a CIN group. After 14 days, serum creatinine returned to the baseline level, but urinary L-FABP level remained still high. However, urinary L-FABP levels in non-CIN group changed little throughout the experimental period. There are no data available on KIM-1 and contrast nephropathy in patients undergoing cardiac catheterization. However, Linagos et al.Citation[26] reported that N-acetyl-beta-(D)-glucosaminidase activity (NAG) or KIM-1 in combination with the covariates cirrhosis, sepsis, oliguria, and mechanical ventilation yielded an area under the receiver operator characteristic curve of 0.78 (95% CI 0.71–0.84) in predicting the composite outcome in patients with acute renal failure. In our study, we also found that urinary NGAL increased significantly as early as 4 hours after cardiac catheterization. An earlier NGAL rise in serum than in urine may be due to the fact that NGAL was released into the circulation, probably secondary to inflammatory activation of neutrophils initiated by cardiac catheterization. Moreover, as NGAL is increased in atherosclerotic plaques,Citation[27] it might be also released into the circulation during the cardiac catheterization. In our previous study, we reported that hypertensive patients had higher serum NGAL than normotensive ones.Citation[28] The findings that diabetics had significantly higher baseline serum but not urinary NGAL may speak in favor of this explanation. A rise in NGAL, despite non-significant changes in creatinine, may be due to renal injury and/or an inflammatory component of NGAL. Herget-Rosenthal et al.Citation[29] reported that serum cystatin C is a useful detection marker of acute kidney failure and might detect it one or two days earlier than creatinine. Rickli et al.Citation[30] observed that the rise in cystatin C achieved a maximum at 24 h after the application of the contrast agent. Therefore, in our study, we simultaneously assessed NGAL, serum cystatin C, creatinine, and eGFR, and confirmed their findings. It should be stressed that NGAL correlated with both cystatin C and creatinine. In our study, NGAL increased prior to rise in serum cystatin C in both groups of patients. In diabetic patients, cystatin C was significantly higher 48 hours after PCI. However, cystatin C is a better marker of GFR than serum creatinine, though it is not a biomarker of kidney injury. Moreover, especially at a point where nobody knows if cystatin C is really superior to creatinine levels in detection of clinical sufficient contrast nephropathy,Citation[31] the search for new and sensitive biomarkers is necessary to meet clinical needs.
Despite many reports on CIN and risk factors, only a few address the problem of diabetic patients, and none of them touch the prevalence of CIN and new biomarkers in low-risk diabetic population (i.e., with normal serum creatinine). In 11,141 consecutive patients from northern New England undergoing PCI without dialysis, preprocedural creatinine (37%), congestive heart failure (24%), and diabetes (15%) accounted for 76% of the predictive ability of the model of serious renal dysfunction.Citation[32] In 936 Chinese patients scheduled for elective PCI, the incidence of CIN was more common in the abnormal group (serum creatinine > 1.5 mg/dL) than in the normal group (6.52% vs. 37.68%, p < 0.001).Citation[33] Predictors of CIN were as follows: age ≥ 70, contrast volume ≥ 320 ml, diabetes mellitus, and peripheral arterial disease. Nikolsky et al.Citation[34] investigated the impact of CKD on prognosis of patients with diabetes (1575) who underwent PCI. CIN was found in 15% of patients without CKD versus 27% of those with CKD (baseline serum creatinine >1.5 mg/dL or estimated glomerular filtration rate <60 mL/min/1.73 m2). In our diabetic patients, prevalence of CIN was similar, 14%. However, we focused on new biomarkers to predict CIN in diabetic population. The presence of diabetes, in addition to increasing age, GFR lower than 90 mL/min, and history of congestive heart failure, were significant predictors of increased mortality in the study of Inrig et al.Citation[35] published recently.
The strength of our study is its prospective design, simultaneous measurement of urinary, serum NGAL, cystatin C, urinary KIM-1, L-FABP, and IL-18 (up to 48 hours after the procedure) on low-risk population. The main limitation is that this is a single-center study, on a population of 70 patients in each group.
Our findings may have important implications for the clinical management of patients undergoing cardiac catheterization. The “window of opportunity” is narrow in contrast nephropathy, and time is limited to introduce proper treatment after initiating insult, particularly when patients are discharged within 24 hours after the procedure. In the perspective of the development of urinaryCitation[36] or whole blood/plasma assaysCitation[37] of NGAL estimation, the new tool for assessment of kidney function would be available. The assay for the whole blood or plasma is easy with quantitative results available in 15 minutes and requires only microliter sample.Citation[37] Urinary assay requires only 150 μl of urine, and results are available within 35 minutes.Citation[32] It could be particularly useful in patients with impaired kidney function, a population particularly prone to rapid deterioration of renal function. It might enable prompt intervention in this clinical setting.
ACKNOWLEDGMENTS
The authors report no conflict of interests.
REFERENCES
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053.
- Parving HH, Hommel E, Mathiesen E, Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed). 1988;296:156–160.
- Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79:211–219.
- Parikh CN, Deavarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(4 Suppl.):S159–S165.
- Mori K, Lee HT, Rapoport D, Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621.
- Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systemic review. Kindey Int. 2008;73:1008–1016.
- Kato K, Sato N, Yamamoto T, Iwasaki YK, Tanaka K, Mizuno K. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circ J. 2008;72:1499–1505.
- Gruberg L, Mintz GS, Mehran R, The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–1548.
- Rihal CS, Textor SC, Grill DE, Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Jeliffe RW. Creatinine clearance: Bedside estimate. Arch Intern Med. 1973;79:604–605.
- Tommaso CL. Contrast-induced nephrotoxicity in patients undergoing cardiac catheterization. Cathet Cardiovasc Diagn. 1994;31:316–321.
- Parfrey PS, Griffiths SM, Barrett BJ, Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149.
- Asif A, Epstein M. Prevention of radiocontrast-induced nephropathy. Am J Kidney Dis. 2004;44:12–24.
- Mishra J, Dent C, Tarabishi R, Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238.
- Detrenis S, Meschi M, Musini S, Savazzi G. Lights and shadows on the pathogenesis of contrast-induced nephropathy: State of the art. Nephrol Dial Transplant. 2005;20:1542–1550.
- King SBIII, Smith SCJr, Hirshfeld JWJr, 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–265.
- Manske CL, Sprafka JM, Strong JH, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–620.
- Hirsch R, Dent C, Pfriem H, NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095.
- Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181.
- Parikh CR, Mishra J, Thiessen-Philbrook H, Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203.
- Bulent Gul CB, Gullulu M, Oral B, Urinary IL-18: A marker of contrast-induced nephropathy following percutaneous coronary intervention?. Clin Biochem. 2008;41:544–547.
- Kato K, Sato N, Yamamoto T, Iwasaki YK, Tanaka K, Mizuno K. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circ J. 2008;72:1499–1505.
- Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47:439–444.
- Liangos O, Perianayagam MC, Vaidya VS, Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912.
- Hemdahl AL, Gabrielsen A, Zhu C, Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–142.
- Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Pawlak K, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in hypertensive and normotensive patients with coronary artery disease. Nephrology (Carlton). 2008;13:153–156.
- Herget-Rosenthal S, Marggraf G, Husing J, Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;6:1115–1122.
- Rickli H, Benou K, Ammann P, Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin Nephrol. 2004;61:98–102.
- Rehman T, Fought J, Solomon R. N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD patients. Clin J Am Soc Nephrol. 2008;3:1610–1614.
- Brown JR, DeVries JT, Piper WD, Northern New England Cardiovascular Disease Study Group. Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am Heart J. 2008;155:260–266.
- Chen SL, Zhang J, Yei F, Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: A prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol. 2008;126:407–413.
- Nikolsky E, Mehran R, Turcot D, Impact of chronic kidney disease on prognosis of patients with diabetes mellitus treated with percutaneous coronary intervention. Am J Cardiol. 2004;94:300–305.
- Inrig JK, Patel UD, Briley LP, Mortality, kidney disease and cardiac procedures following acute coronary syndrome. Nephrol Dial Transplant. 2008;23:934–940.
- Nickolas TL, O'Rourke MJ, Yang J, Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819.
- Dent CL, Ma Q, Dastrala S, Plasma NGAL predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care. 2007;11:R127.